Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons
- PMID: 19321767
- PMCID: PMC2712668
- DOI: 10.1523/JNEUROSCI.0431-09.2009
Thyrotropin-releasing hormone increases behavioral arousal through modulation of hypocretin/orexin neurons
Abstract
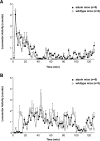
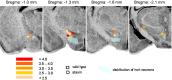
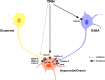
Thyrotropin-releasing hormone (TRH) has previously been shown to promote wakefulness and to induce arousal from hibernation. Expression of TRH-R1 (TRH receptor 1) is enriched in the tuberal and lateral hypothalamic area (LHA), brain regions in which the hypocretin/orexin (Hcrt) cells are located. Because the Hcrt system is implicated in sleep/wake control, we hypothesized that TRH provides modulatory input to the Hcrt cells. In vitro electrophysiological studies showed that bath application of TRH caused concentration-dependent membrane depolarization, decreased input resistance, and increased firing rate of identified Hcrt neurons. In the presence of tetrodotoxin, TRH induced inward currents that were associated with a decrease in frequency, but not amplitude, of miniature postsynaptic currents (PSCs). Ion substitution experiments suggested that the TRH-induced inward current was mediated in part by Ca(2+) influx. Although TRH did not significantly alter either the frequency or amplitude of spontaneous excitatory PSCs, TRH (100 nm) increased the frequency of spontaneous inhibitory PSCs by twofold without affecting the amplitude of these events, indicating increased presynaptic GABA release onto Hcrt neurons. In contrast, TRH significantly reduced the frequency, but not amplitude, of miniature excitatory PSCs without affecting miniature inhibitory PSC frequency or amplitude, indicating that TRH also reduces the probability of glutamate release onto Hcrt neurons. When injected into the LHA, TRH increased locomotor activity in wild-type mice but not in orexin/ataxin-3 mice in which the Hcrt neurons degenerate postnatally. Together, these results are consistent with the hypothesis that TRH modulates behavioral arousal, in part, through the Hcrt system.
Figures

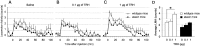
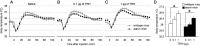
Similar articles
-
Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia.J Clin Invest. 2008 Jul;118(7):2471-81. doi: 10.1172/JCI35115. J Clin Invest. 2008. PMID: 18551194 Free PMC article.
-
Direct and indirect control of orexin/hypocretin neurons by glycine receptors.J Physiol. 2011 Feb 1;589(Pt 3):639-51. doi: 10.1113/jphysiol.2010.198457. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135047 Free PMC article.
-
Lack of hypocretin attenuates behavioral changes produced by glutamatergic activation of the perifornical-lateral hypothalamic area.Sleep. 2014 May 1;37(5):1011-20. doi: 10.5665/sleep.3680. Sleep. 2014. PMID: 24790280 Free PMC article.
-
Local network regulation of orexin neurons in the lateral hypothalamus.Am J Physiol Regul Integr Comp Physiol. 2011 Sep;301(3):R572-80. doi: 10.1152/ajpregu.00674.2010. Epub 2011 Jun 22. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21697524 Review.
-
Hypocretin/orexin in arousal and stress.Brain Res. 2010 Feb 16;1314:91-102. doi: 10.1016/j.brainres.2009.09.019. Epub 2009 Sep 11. Brain Res. 2010. PMID: 19748490 Free PMC article. Review.
Cited by
-
Thyrotropin-Releasing Hormone and Food Intake in Mammals: An Update.Metabolites. 2024 May 26;14(6):302. doi: 10.3390/metabo14060302. Metabolites. 2024. PMID: 38921437 Free PMC article. Review.
-
Effects of the domestic thyroid stimulating hormone receptor (TSHR) variant on the hypothalamic-pituitary-thyroid axis and behavior in chicken.Genetics. 2021 Mar 3;217(1):1-9. doi: 10.1093/genetics/iyaa050. Genetics. 2021. PMID: 33683367 Free PMC article.
-
The effects of amphetamine injections on feeding behavior and the brain expression of orexin, CART, tyrosine hydroxylase (TH) and thyrotropin releasing hormone (TRH) in goldfish (Carassius auratus).Fish Physiol Biochem. 2013 Aug;39(4):979-91. doi: 10.1007/s10695-012-9756-4. Epub 2012 Dec 11. Fish Physiol Biochem. 2013. PMID: 23229307
-
Hypothyroidism compromises hypothalamic leptin signaling in mice.Mol Endocrinol. 2013 Apr;27(4):586-97. doi: 10.1210/me.2012-1311. Epub 2013 Mar 21. Mol Endocrinol. 2013. PMID: 23518925 Free PMC article.
-
Unmet needs of patients with narcolepsy: perspectives on emerging treatment options.Nat Sci Sleep. 2015 May 22;7:51-61. doi: 10.2147/NSS.S56077. eCollection 2015. Nat Sci Sleep. 2015. PMID: 26045680 Free PMC article. Review.
References
-
- Boler J, Enzmann F, Folkers K, Bowers CY, Schally AV. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem Biophys Res Commun. 1969;37:705–710. - PubMed
-
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
