Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate
- PMID: 19321781
- PMCID: PMC2707781
- DOI: 10.1523/JNEUROSCI.5842-08.2009
Cisplatin-induced hair cell death requires STAT1 and is attenuated by epigallocatechin gallate
Abstract
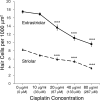
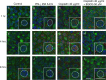
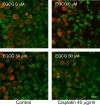
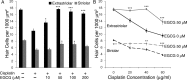
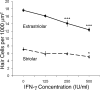
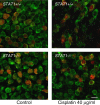
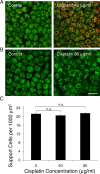
Cisplatin is a chemotherapy drug that frequently causes auditory impairment due to the death of mechanosensory hair cells. Cisplatin ototoxicity may result from oxidative stress, DNA damage, and inflammatory cytokines. The transcription factor STAT1, an important mediator of cell death, can regulate all of these processes in other cell types. We used cultured utricles from mature Swiss Webster mice to investigate the role of STAT1 in cisplatin-induced hair cell death. We show that STAT1 phosphorylation is an early event in both hair cells and support cells after exposure of utricles to cisplatin. STAT1 phosphorylation peaked after 4 h of cisplatin exposure and returned to control levels by 8 h of exposure. The STAT1 inhibitor epigallocatechin gallate (EGCG) attenuated STAT1 phosphorylation in cisplatin-treated utricles and resulted in concentration-dependent increases in hair cell survival at 24 h postexposure. Furthermore, we show that utricular hair cells from STAT1-deficient mice are resistant to cisplatin toxicity. EGCG failed to provide additional protection from cisplatin in STAT1-deficient mice, further supporting the hypothesis that the protective effects of EGCG are due to its inhibition of STAT1. Treatment with IFN-gamma, which also causes STAT1 activation, also induced hair cell death in wild-type but not STAT1-deficient mice. These results show that STAT1 is required for maximal cisplatin-induced hair cell death in the mouse utricle and suggest that treatment with EGCG may be a useful strategy for prevention of cisplatin ototoxicity.
Figures

References
-
- Barton C, Davies D, Balkwill F, Burke F. Involvement of both intrinsic and extrinsic pathways in IFN-γ-induced apoptosis that are enhanced with cisplatin. Eur J Cancer. 2005;41:1474–1486. - PubMed
-
- Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. - PubMed
-
- Bodmer D, Brors D, Bodmer M, Pak K, Ryan AF. Fas ligand expression in the organ of Corti. Audiol Neurootol. 2003;8:243–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
