Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol
- PMID: 19322169
- PMCID: PMC2822461
- DOI: 10.1038/npp.2009.31
Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol
Abstract
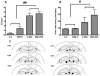
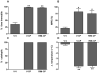
It is firmly established that the hippocampus, a brain region implicated in spatial learning, episodic memory, and consolidation, contains a high concentration of CB(1) receptors. Moreover, systemic and intrahippocampal administration of cannabinoid agonists have been shown to impair hippocampal-dependent memory tasks. However, the degree to which CB(1) receptors in the hippocampus play a specific functional role in the memory disruptive effects of marijuana or its primary psychoactive constituent Delta(9)-tetrahydrocannabinol (Delta(9)-THC) is unknown. This study was designed to determine whether hippocampal CB(1) receptors play a functional role in the memory disruptive effects of systemically administered cannabinoids, using the radial arm maze, a well characterized rodent model of working memory. Male Sprague-Dawley rats were implanted with bilateral cannulae aimed at the CA1 region of the dorsal hippocampus. The CB(1) receptor antagonist, rimonabant, was delivered into the hippocampus before to a systemic injection of either Delta(9)-THC or the potent cannabinoid analog, CP-55,940. Strikingly, intrahippocampal administration of rimonabant completely attenuated the memory disruptive effects of both cannabinoids in the radial arm maze task, but did not affect other pharmacological properties of cannabinoids, as assessed in the tetrad assay (that is, hypomotility, analgesia, catalepsy, and hypothermia). Infusions of rimonabant just dorsal or ventral to the hippocampus did not prevent Delta(9)-THC-induced memory impairment, indicating that its effects on mnemonic function were regionally selective. These findings provide compelling evidence in support of the view that hippocampal CB(1) receptors play a necessary role in the memory disruptive effects of marijuana.
Conflict of interest statement
Figures



References
-
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther. 1997;282:1526–1532. - PubMed
-
- Compton D, Aceto M, Lowe J, Martin B. In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of Δ9-tetrahdrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther. 1996;277:586–594. - PubMed
-
- Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacological profile of a series of bicyclic cannabinoid analogs: Classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260:201–209. - PubMed
-
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharm Exp Ther. 1941;72:74–79.
-
- Deadwyler SA, Goonawardena AV, Hampson RE. Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol. 2007;18:571–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

