Endogenous gamma-aminobutyric acid modulates tonic guinea pig airway tone and propofol-induced airway smooth muscle relaxation
- PMID: 19322939
- PMCID: PMC3608685
- DOI: 10.1097/aln.0b013e31819c44e1
Endogenous gamma-aminobutyric acid modulates tonic guinea pig airway tone and propofol-induced airway smooth muscle relaxation
Abstract
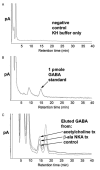
Background: Emerging evidence indicates that an endogenous autocrine/paracrine system involving gamma-aminobutyric acid (GABA) is present in airways. GABAA channels, GABAB receptors, and the enzyme that synthesizes GABA have been identified in airway epithelium and smooth muscle. However, the endogenous ligand itself, GABA, has not been measured in airway tissues. The authors sought to demonstrate that GABA is released in response to contractile agonists and tonically contributes a prorelaxant component to contracted airway smooth muscle.
Methods: The amount and cellular localization of GABA in upper guinea pig airways under resting and contracted tone was determined by high pressure liquid chromatography and immunohistochemistry, respectively. The contribution that endogenous GABA imparts on the maintenance of airway smooth muscle acetylcholine-induced contraction was assessed in intact guinea pig airway tracheal rings using selective GABAA antagonism (gabazine) under resting or acetylcholine-contracted conditions. The ability of an allosteric agent (propofol) to relax a substance P-induced relaxation in an endogenous GABA-dependent manner was assessed.
Results: GABA levels increased and localized to airway smooth muscle after contractile stimuli in guinea pig upper airways. Acetylcholine-contracted guinea pig tracheal rings exhibited an increase in contracted force upon addition of the GABAA antagonist gabazine that was subsequently reversed by the addition of the GABAA agonist muscimol. Propofol dose-dependently relaxed a substance P contraction that was blocked by gabazine.
Conclusion: These studies demonstrate that GABA is endogenously present and increases after contractile stimuli in guinea pig upper airways and that endogenous GABA contributes a tonic prorelaxant component in the maintenance of airway smooth muscle tone.
Figures









Comment in
-
Gamma-aminobutyric acid: something old, something new for bronchodilation.Anesthesiology. 2009 Apr;110(4):696-7. doi: 10.1097/ALN.0b013e31819c4291. Anesthesiology. 2009. PMID: 19276969 No abstract available.
References
-
- Eder W, Ege MJ, von ME. The asthma epidemic. N Engl J Med. 2006;355:2226–35. - PubMed
-
- Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc. 2008;5:23–31. - PubMed
-
- Rooke GA, Choi JH, Bishop MJ. The effect of isoflurane, halothane, sevoflurane, and thiopental/nitrous oxide on respiratory system resistance after tracheal intubation. Anesthesiology. 1997;86:1294–9. - PubMed
-
- Eames WO, Rooke GA, Wu RS, Bishop MJ. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. 1996;84:1307–11. - PubMed
-
- Pizov R, Brown RH, Weiss YS, Baranov D, Hennes H, Baker S, Hirshman CA. Wheezing during induction of general anesthesia in patients with and without asthma: a randomized blinded trial. Anesthesiology. 1995;82:1111–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

