Coupled growth and division of model protocell membranes
- PMID: 19323552
- PMCID: PMC2669828
- DOI: 10.1021/ja900919c
Coupled growth and division of model protocell membranes
Abstract
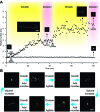
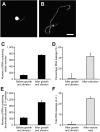
The generation of synthetic forms of cellular life requires solutions to the problem of how biological processes such as cyclic growth and division could emerge from purely physical and chemical systems. Small unilamellar fatty acid vesicles grow when fed with fatty acid micelles and can be forced to divide by extrusion, but this artificial division process results in significant loss of protocell contents during each division cycle. Here we describe a simple and efficient pathway for model protocell membrane growth and division. The growth of large multilamellar fatty acid vesicles fed with fatty acid micelles, in a solution where solute permeation across the membranes is slow, results in the transformation of initially spherical vesicles into long thread-like vesicles, a process driven by the transient imbalance between surface area and volume growth. Modest shear forces are then sufficient to cause the thread-like vesicles to divide into multiple daughter vesicles without loss of internal contents. In an environment of gentle shear, protocell growth and division are thus coupled processes. We show that model protocells can proceed through multiple cycles of reproduction. Encapsulated RNA molecules, representing a primitive genome, are distributed to the daughter vesicles. Our observations bring us closer to the laboratory synthesis of a complete protocell consisting of a self-replicating genome and a self-replicating membrane compartment. In addition, the robustness and simplicity of this pathway suggests that similar processes might have occurred under the prebiotic conditions of the early Earth.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

