Bioorganic chemistry. A natural reunion of the physical and life sciences
- PMID: 19323569
- PMCID: PMC2680001
- DOI: 10.1021/jo900183c
Bioorganic chemistry. A natural reunion of the physical and life sciences
Abstract
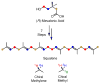
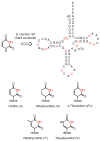
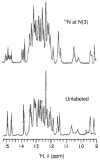
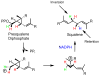
Organic substances were conceived as those found in living organisms. Although the definition was soon broadened to include all carbon-containing compounds, naturally occurring molecules have always held a special fascination for organic chemists. From these beginnings, molecules from nature were indespensible tools as generations of organic chemists developed new techniques for determining structures, analyzed the mechanisms of reactions, explored the effects conformation and stereochemistry on reactions, and found challenging new targets to synthesize. Only recently have organic chemists harnessed the powerful techniques of organic chemistry to study the functions of organic molecules in their biological hosts, the enzymes that synthesize molecules and the complex processes that occur in a cell. In this Perspective, I present a personal account of my entree into bioorganic chemistry as a physical organic chemist and subsequent work to understand the chemical mechanisms of enzyme-catalyzed reactions, to develop techniques to identify and assign hydrogen bonds in tRNAs through NMR studies with isotopically labeled molecules, and to study how structure determines function in biosynthetic enzymes with proteins obtained by genetic engineering.
Figures















References
-
- Jorpes JE. Jac Berzelius: his life and work. 1. Univ. Calif. Press; Berkeley: 1970.
-
- Nobel Lectures, Chemistry 1901–1921. World Scientific; River Edge, NJ: 1999. pp. 175–196.
-
- Meerwein H, van Emster K. Ber. 1922;55:2500.
-
- Woodward RB, Sondheimer F, Heusler K, McLamore WM. J Am Chem Soc. 1952;74:4223–4251.
-
- Barton DHR. Science. 1970;169:539–544. - PubMed
Publication types
MeSH terms
Substances
Personal name as subject
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

