Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice
- PMID: 19323847
- PMCID: PMC2673217
- DOI: 10.1186/1756-6606-2-8
Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice
Abstract
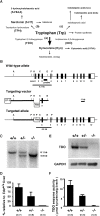
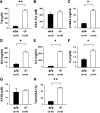
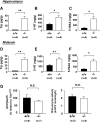
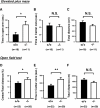
Although nutrients, including amino acids and their metabolites such as serotonin (5-HT), are strong modulators of anxiety-related behavior, the metabolic pathway(s) responsible for this physiological modulation is not fully understood. Regarding tryptophan (Trp), the initial rate-limiting enzymes for the kynurenine pathway of tryptophan metabolism are tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO). Here, we generated mice deficient for tdo (Tdo(-/-)). Compared with wild-type littermates, Tdo(-/-) mice showed increased plasma levels of Trp and its metabolites 5-hydroxyindoleacetic acid (5-HIAA) and kynurenine, as well as increased levels of Trp, 5-HT and 5-HIAA in the hippocampus and midbrain. These mice also showed anxiolytic modulation in the elevated plus maze and open field tests, and increased adult neurogenesis, as evidenced by double staining of BrdU and neural progenitor/neuronal markers. These findings demonstrate a direct molecular link between Trp metabolism and neurogenesis and anxiety-related behavior under physiological conditions.
Figures


References
-
- Peters JC. Tryptophan nutrition and metabolism: an overview. Adv Exp Med Biol. 1991;294:345–358. - PubMed
-
- Russo S, Kema IP, Fokkema MR, Boon JC, Willemse PH, de Vries EG, den Boer JA, Korf J. Tryptophan as a link between psychopathology and somatic states. Psychosom Med. 2003;65:665–671. doi: 10.1097/01.PSY.0000078188.74020.CC. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

