Subcellular topography of visually driven dendritic activity in the vertebrate visual system
- PMID: 19323998
- PMCID: PMC2892759
- DOI: 10.1016/j.neuron.2009.01.018
Subcellular topography of visually driven dendritic activity in the vertebrate visual system
Abstract
Neural pathways projecting from sensory organs to higher brain centers form topographic maps in which neighbor relationships are preserved from a sending to a receiving neural population. Sensory input can generate compartmentalized electrical and biochemical activity in the dendrites of a receiving neuron. Here, we show that in the developing retinotectal projection of young Xenopus tadpoles, visually driven Ca2+ signals are topographically organized at the subcellular, dendritic scale. Functional in vivo two-photon Ca2+ imaging revealed that the sensitivity of dendritic Ca2+ signals to stimulus location in visual space is correlated with their anatomical position within the dendritic tree of individual neurons. This topographic distribution was dependent on NMDAR activation, whereas global Ca2+ signals were mediated by Ca2+ influx through dendritic, voltage-dependent Ca2+ channels. These findings suggest a framework for plasticity models that invoke local dendritic Ca2+ signaling in the elaboration of neural connectivity and dendrite-specific information storage.
Figures
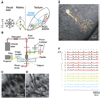
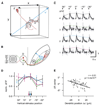

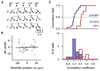
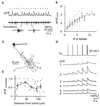

Comment in
-
Caught on film: the secret lives of dendrites in the tadpole optic tectum.Neuron. 2009 Mar 26;61(6):813-4. doi: 10.1016/j.neuron.2009.03.009. Neuron. 2009. PMID: 19323990
References
-
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron. 2003;39:831–842. - PubMed
-
- Baden T, Hedwig B. Neurite-specific Ca2+ dynamics underlying sound processing in an auditory interneurone. J. Neurobiol. 2006;67:68–80. - PubMed
-
- Broser PJ, Schulte R, Lang S, Roth A, Helmchen F, Waters J, Sakmann B, Wittum G. Nonlinear anisotropic diffusion filtering of three-dimensional image data from two-photon microscopy. J. Biomed. Opt. 2004;9:1253–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

