Coactivation of ATM/ERK/NF-kappaB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes
- PMID: 19324081
- PMCID: PMC6759050
- DOI: 10.1016/j.freeradbiomed.2009.03.012
Coactivation of ATM/ERK/NF-kappaB in the low-dose radiation-induced radioadaptive response in human skin keratinocytes
Abstract
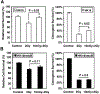
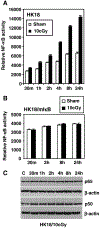
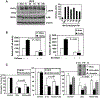
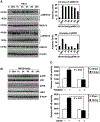
Elucidating the molecular mechanism of the low-dose radiation (LDR)-mediated radioadaptive response is crucial for inventing potential therapeutic approaches to improving normal tissue protection in radiation therapy. ATM, a DNA-damage sensor, is known to activate the stress-sensitive transcription factor NF-kappaB upon exposure to ionizing radiation. This study provides evidence of the cooperative functions of ATM, ERK, and NF-kappaB in inducing a survival advantage through a radioadaptive response as a result of LDR treatment (10 cGy X-rays). By using p53-inhibited human skin keratinocytes, we show that phosphorylation of ATM, MEK, and ERK (but not JNK or p38) is enhanced along with a twofold increase in NF-kappaB luciferase activity at 24 h post-LDR. However, NF-kappaB reporter gene transactivation without a significant enhancement of p65 or p50 protein level suggests that NF-kappaB is activated as a rapid protein response via ATM without involving the transcriptional activation of NF-kappaB subunit genes. A direct interaction between ATM and NF-kappaB p65 is detected in the resting cells and this interaction is significantly increased with LDR treatment. Inhibition of ATM with caffeine, KU-55933, or siRNA or inhibition of the MEK/ERK pathway can block the LDR-induced NF-kappaB activation and eliminate the LDR-induced survival advantage. Altogether, these results suggest a p53-independent prosurvival network involving the coactivation of the ATM, MEK/ERK, and NF-kappaB pathways in LDR-treated human skin keratinocytes, which is absent from mutant IkappaB cells (HK18/mIkappaB), which fail to express NF-kappaB activity.
Figures


References
-
- Kelsey KT; Memisoglu A; Frenkel D; Liber HL Human lymphocytes exposed to low doses of X-rays are less susceptible to radiation-induced mutagenesis. Mutat. Res 263:197–201; 1991. - PubMed
-
- Redpath JL; Short SC; Woodcock M; Johnston PJ Low-dose reduction in transformation frequency compared to unirradiated controls: the role of hyperradiosensitivity to cell death. Radiat. Res 159:433–436; 2003. - PubMed
-
- Spitz DR; Azzam EI; Li JJ; Gius D Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 23:311–322; 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

