Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF
- PMID: 19324301
- PMCID: PMC2666890
- DOI: 10.1016/j.hrthm.2008.12.028
Atrial cellular electrophysiological changes in patients with ventricular dysfunction may predispose to AF
Abstract
Background: Left ventricular systolic dysfunction (LVSD) is a risk factor for atrial fibrillation (AF), but the atrial cellular electrophysiological mechanisms in humans are unclear.
Objective: This study sought to investigate whether LVSD in patients who are in sinus rhythm (SR) is associated with atrial cellular electrophysiological changes that could predispose to AF.
Methods: Right atrial myocytes were obtained from 214 consenting patients in SR who were undergoing cardiac surgery. Action potentials or ion currents were measured using the whole-cell-patch clamp technique.
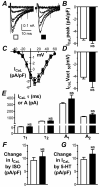
Results: The presence of moderate or severe LVSD was associated with a shortened atrial cellular effective refractory period (ERP) (209 +/- 8 ms; 52 cells, 18 patients vs 233 +/- 7 ms; 134 cells, 49 patients; P <0.05); confirmed by multiple linear regression analysis. The left ventricular ejection fraction (LVEF) was markedly lower in patients with moderate or severe LVSD (36% +/- 4%, n = 15) than in those without LVSD (62% +/- 2%, n = 31; P <0.05). In cells from patients with LVEF <or= 45%, the ERP and action potential duration at 90% repolarization were shorter than in those from patients with LVEF > 45%, by 24% and 18%, respectively. The LVEF and ERP were positively correlated (r = 0.65, P <0.05). The L-type calcium ion current, inward rectifier potassium ion current, and sustained outward ion current were unaffected by LVSD. The transient outward potassium ion current was decreased by 34%, with a positive shift in its activation voltage, and no change in its decay kinetics.
Conclusion: LVSD in patients in SR is independently associated with a shortening of the atrial cellular ERP, which may be expected to contribute to a predisposition to AF.
Figures
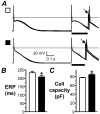
 ) to produce an S2 response of amplitude>80% of S1. Mean±SE ERP (B) and capacity (C) of cells from patients with no LVSD (□; n=134 cells, 49 patients for ERP, and 535 cells, 135 patients for capacity) and moderate/severe LVSD (■; n=52 cells, 18 patients for ERP, and 152 cells, 38 patients for capacity). *=P<0.05 vs □.
) to produce an S2 response of amplitude>80% of S1. Mean±SE ERP (B) and capacity (C) of cells from patients with no LVSD (□; n=134 cells, 49 patients for ERP, and 535 cells, 135 patients for capacity) and moderate/severe LVSD (■; n=52 cells, 18 patients for ERP, and 152 cells, 38 patients for capacity). *=P<0.05 vs □.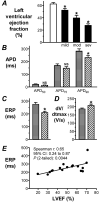

Comment in
-
Substrate for development of atrial fibrillation in patients with congestive heart failure: are we close to the answer?Heart Rhythm. 2009 Apr;6(4):452-3. doi: 10.1016/j.hrthm.2009.01.014. Epub 2009 Jan 18. Heart Rhythm. 2009. PMID: 19246251 No abstract available.
References
-
- Neuberger H-R, Mewis C, Van Veldhuisen DJ, et al. Management of atrial fibrillation in patients with heart failure. Eur Heart J. 2007;28:2568–2577. - PubMed
-
- Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc Res. 2001;52:226–235. - PubMed
-
- Koumi S, Arentzen CE, Backer CL, et al. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994;90:2213–2224. - PubMed
-
- Schreieck J, Wang YG, Kalra B, et al. Differential rate dependence of action potentials, calcium inward and transient outward current in atrial myocytes of patients with and without heart failure. Circulation. 1998;98:611. Abstract.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

