An experimental test of the independent action hypothesis in virus-insect pathosystems
- PMID: 19324752
- PMCID: PMC2677602
- DOI: 10.1098/rspb.2009.0064
An experimental test of the independent action hypothesis in virus-insect pathosystems
Abstract
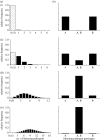
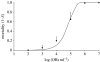
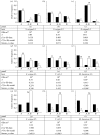
The 'independent action hypothesis' (IAH) states that each pathogen individual has a non-zero probability of causing host death and that pathogen individuals act independently. IAH has not been rigorously tested. In this paper, we (i) develop a probabilistic framework for testing IAH and (ii) demonstrate that, in two out of the six virus-insect pathosystems tested, IAH is supported by the data. We first show that IAH inextricably links host survivorship to the number of infecting pathogen individuals, and develop a model to predict the frequency of single- and dual-genotype infections when a host is challenged with a mixture of two genotypes. Model predictions were tested using genetically marked, near-identical baculovirus genotypes, and insect larvae from three host species differing in susceptibility. Observations in early-instar larvae of two susceptible host species support IAH, but observations in late-instar larvae of susceptible host species and larvae of a less susceptible host species were not in agreement with IAH. Hence the model is experimentally supported only in pathosystems in which the host is highly susceptible. We provide, to our knowledge, the first qualitative experimental evidence that, in such pathosystems, the action of a single virion is sufficient to cause disease.
Figures

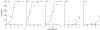
References
-
- Ali A., Li H.Y., Schneider W.L., Sherman D.J., Gray S., Smith D., Roossinck M.J. Analysis of genetic bottlenecks during horizontal transmission of cucumber mosaic virus. J. Virol. 2006;80:8345–8350. doi:10.1128/JVI.00568-06 - DOI - PMC - PubMed
-
- Ben-Ami F., Regoes R.R., Ebert D. A quantitative test of the relationship between parasite dose and infection probability across different host–parasite combinations. Proc. R. Soc. B. 2008;275:853–859. doi:10.1098/rspb.2007.1544 - DOI - PMC - PubMed
-
- Bianchi F., Snoeijing I., Van der Werf W., Mans R.M.W., Smits P.H., Vlak J.M. Biological activity of SeMNPV, AcMNPV, and three AcMNPV deletion mutants against Spodoptera exigua larvae (Lepidoptera: Noctuidae) J. Invertebr. Pathol. 2000;75:28–35. doi:10.1006/jipa.1999.4907 - DOI - PubMed
-
- Bianchi F., Vlak J.M., Rabbinge R., Van der Werf W. Biological control of beet armyworm, Spodoptera exigua, with baculoviruses in greenhouses: development of a comprehensive process-based model. Biol. Control. 2002;23:35–46. doi:10.1006/bcon.2001.0989 - DOI
-
- Burden J.P., Griffiths C.M., Cory J.S., Smith P., Sait S.M. Vertical transmission of sublethal granulovirus infection in the Indian meal moth, Plodia interpunctella. Mol. Ecol. 2002;11:547–555. doi:10.1046/j.0962-1083.2001.01439.x - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

