T cell receptor-mediated activation of p38{alpha} by mono-phosphorylation of the activation loop results in altered substrate specificity
- PMID: 19324872
- PMCID: PMC2708844
- DOI: 10.1074/jbc.M901004200
T cell receptor-mediated activation of p38{alpha} by mono-phosphorylation of the activation loop results in altered substrate specificity
Abstract
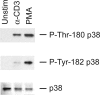
p38 MAPKs are typically activated by upstream MAPK kinases that phosphorylate a Thr-X-Tyr motif in the activation loop. An exception is the T cell antigen receptor signaling pathway, which bypasses the MAPK cascade and activates p38alpha and p38beta by phosphorylation of Tyr-323 and subsequent autophosphorylation of the activation loop. Here we show that, unlike the classic MAPK cascade, the alternative pathway results primarily in mono-phosphorylation of the activation loop residue Thr-180. Recombinant mono-phosphorylated and dual phosphorylated p38alpha differed widely with regard to activity and substrate preference. Altered substrate specificity was reproduced in T cells in which p38 was activated by the alternative or classical MAPK pathways. These findings suggest that T cells have evolved a mechanism to utilize p38 in a specialized manner independent of and distinct from the classical p38 MAPK signaling cascade.
Figures
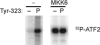




References
-
- Kyriakis J. M., Avruch J. ( 2001) Physiol. Rev. 81, 807– 869 - PubMed
-
- Engelberg D. ( 2004) Semin. Cancer Biol. 14, 271– 282 - PubMed
-
- Lee J. C., Kumar S., Griswold D. E., Underwood D. C., Votta B. J., Adams J. L. ( 2000) Immunopharmacology 47, 185– 201 - PubMed
-
- Li Z., Jiang Y., Ulevitch R. J., Han J. ( 1996) Biochem. Biophys. Res. Commun. 228, 334– 340 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

