Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling
- PMID: 19324938
- PMCID: PMC2682666
- DOI: 10.2337/db08-1697
Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling
Abstract
Objective: Glycogen synthase kinase (GSK)-3beta plays an important role in cardiomyopathies. Cardiac-specific metallothionein-overexpressing transgenic (MT-TG) mice were highly resistant to diabetes-induced cardiomyopathy. Therefore, we investigated whether metallothionein cardiac protection against diabetes is mediated by inactivation of GSK-3beta.
Research design and methods: Diabetes was induced with streptozotocin in both MT-TG and wild-type mice. Changes of energy metabolism-related molecules, lipid accumulation, inflammation, nitrosative damage, and fibrotic remodeling were examined in the hearts of diabetic mice 2 weeks, 2 months, and 5 months after the onset of diabetes with Western blotting, RT-PCR, and immunohistochemical assays.
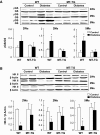
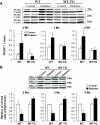
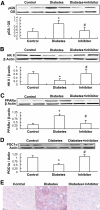
Results: Activation (dephosphorylation) of GSK-3beta was evidenced in the hearts of wild-type diabetic mice but not MT-TG diabetic mice. Correspondingly, cardiac glycogen synthase phosphorylation, hexokinase II, PPARalpha, and PGC-1alpha expression, which mediate glucose and lipid metabolisms, were significantly changed along with cardiac lipid accumulation, inflammation (TNF-alpha, plasminogen activator inhibitor 1 [PAI-1], and intracellular adhesion molecule 1 [ICAM-1]), nitrosative damage (3-nitrotyrosin accumulation), and fibrosis in the wild-type diabetic mice. The above pathological changes were completely prevented either by cardiac metallothionein in the MT-TG diabetic mice or by inhibition of GSK-3beta activity in the wild-type diabetic mice with a GSK-3beta-specific inhibitor.
Conclusions: These results suggest that activation of GSK-3beta plays a critical role in diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Metallothionein inactivation of GSK-3beta plays a critical role in preventing diabetic cardiomyopathy.
Figures



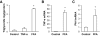


References
-
- Ye G, Metreveli NS, Ren J, Epstein PN: Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes 2003; 52: 777– 783 - PubMed
-
- Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ: Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 2005; 54: 1829– 1837 - PubMed
-
- Fang CX, Dong F, Ren BH, Epstein PN, Ren J: Metallothionein alleviates cardiac contractile dysfunction induced by insulin resistance: role of Akt phosphorylation, PTB1B, PPARgamma and c-Jun. Diabetologia 2005; 48: 2412– 2421 - PubMed
-
- Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ: Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 2006; 48: 1688– 1697 - PubMed
-
- Dong F, Li Q, Sreejayan N, Nunn JM, Ren J: Metallothionein prevents high-fat diet–induced cardiac contractile dysfunction: role of peroxisome proliferator–activated receptor γ coactivator 1α and mitochondrial biogenesis. Diabetes 2007; 56: 2201– 2212 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

