Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers
- PMID: 19324974
- PMCID: PMC2695496
- DOI: 10.1164/rccm.200810-1584OC
Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers
Abstract
Rationale: Simvastatin inhibits inflammatory responses in vitro and in murine models of lung inflammation in vivo. As simvastatin modulates a number of the underlying processes described in acute lung injury (ALI), it may be a potential therapeutic option.
Objectives: To investigate in vivo if simvastatin modulates mechanisms important in the development of ALI in a model of acute lung inflammation induced by inhalation of lipopolysaccharide (LPS) in healthy human volunteers.
Methods: Thirty healthy subjects were enrolled in a double-blind, placebo-controlled study. Subjects were randomized to receive 40 mg or 80 mg of simvastatin or placebo (n = 10/group) for 4 days before inhalation of 50 microg LPS. Measurements were performed in bronchoalveolar lavage fluid (BALF) obtained at 6 hours and plasma obtained at 24 hours after LPS challenge. Nuclear translocation of nuclear factor-kappaB (NF-kappaB) was measured in monocyte-derived macrophages.
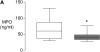
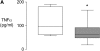
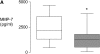
Measurements and main results: Pretreatment with simvastatin reduced LPS-induced BALF neutrophilia, myeloperoxidase, tumor necrosis factor-alpha, matrix metalloproteinases 7, 8, and 9, and C-reactive protein (CRP) as well as plasma CRP (all P < 0.05 vs. placebo). There was no significant difference between simvastatin 40 mg and 80 mg. BALF from subjects post-LPS inhalation induced a threefold up-regulation in nuclear NF-kappaB in monocyte-derived macrophages (P < 0.001); pretreatment with simvastatin reduced this by 35% (P < 0.001).
Conclusions: Simvastatin has antiinflammatory effects in the pulmonary and systemic compartment in humans exposed to inhaled LPS.
Figures









Comment in
-
Simvastatin as a potential therapeutic for acute respiratory distress syndrome.Am J Respir Crit Care Med. 2009 Nov 15;180(10):1031; author reply 1031-2. doi: 10.1164/ajrccm.180.10.1031a. Am J Respir Crit Care Med. 2009. PMID: 19897776 No abstract available.
References
-
- Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA. Alveolar macrophages mediate epithelial barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L1191–L1198. - PubMed
-
- O'Kane CM, Frank JA, McAuley DF. Matrix metalloproteases; a potential role in the pathogenesis of the acute respiratory distress syndrome. In J. L. Vincent, editor. 24th yearbook of intensive care and emergency medicine. Berlin: Springer-Verlag; 2004. pp. 287–300.
-
- Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, et al. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 1999;162:3653–3662. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

