Signaling by hypoxia-inducible factors is critical for ovulation in mice
- PMID: 19325003
- PMCID: PMC2703551
- DOI: 10.1210/en.2008-0948
Signaling by hypoxia-inducible factors is critical for ovulation in mice
Abstract
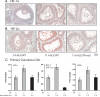
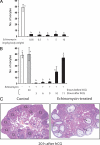
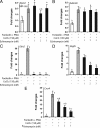
The steroid hormone progesterone, acting via its nuclear receptor, is a major regulator of the process of ovulation. Female mice lacking progesterone receptor (PGR) exhibit an anovulatory phenotype due to failure in follicular rupture. To identify the PGR-regulated pathways that control ovulation, we analyzed global changes in gene expression in the ovaries of wild-type and Pgr-null mice subjected to gonadotropin-induced superovulation. Our analysis uncovered several genes whose expression was reduced in the Pgr-null ovaries compared with the wild-type ovaries immediately preceding ovulation. Interestingly, these genes included three hypoxia-inducible factors (HIFs): HIF-1 alpha, HIF-2 alpha, and HIF-1 beta. These transcription factors form alphabeta-heterodimers, which regulate the transcription of specific cellular genes, thereby mediating adaptive response of the tissue to low-oxygen levels. We observed that the expression of mRNAs and proteins corresponding to HIF-1 alpha, HIF-2 alpha, and HIF-1 beta was induced in a PGR-dependent manner, specifically in the granulosa cells of the preovulatory follicles. Inhibition of the HIF transcriptional activity by echinomycin, a small-molecule inhibitor that suppresses the binding of HIF alphabeta-heterodimers to target genes, blocked ovulation by preventing the rupture of the preovulatory follicles. Echinomycin specifically inhibited the expression of genes that are known regulators of ovulation, such as a disintegrin and metalloproteinase with thrombospondin-like motifs-1 and endothelin-2. Furthermore, echinomycin reduced the expression of vascular endothelial growth factor A, a key factor controlling vascularization/angiogenesis during ovulation. Collectively, these findings unveiled a novel ovarian role for the HIF transcription factors during the ovulatory period in mice.
Figures



References
-
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 - PubMed
-
- Richards JS, Russell DL, Ochsner S, Espey LL 2002 Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64:69–92 - PubMed
-
- Park OK, Mayo KE 1991 Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5:967–978 - PubMed
-
- Li X, Lonard DM, O'Malley BW 2004 A contemporary understanding of progesterone receptor function. Mech Ageing Dev 125:669–678 - PubMed
-
- Loutradis D, Bletsa R, Aravantinos L, Kallianidis K, Michalas S, Psychoyos A 1991 Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum Reprod 6:1238–1240 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

