Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection
- PMID: 19325114
- PMCID: PMC2753492
- DOI: 10.1126/science.1168676
Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection
Abstract
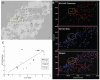
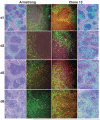
In the early stages of viral infection, outcomes depend on a race between expansion of infection and the immune response generated to contain it. We combined in situ tetramer staining with in situ hybridization to visualize, map, and quantify relationships between immune effector cells and their targets in tissues. In simian immunodeficiency virus infections in macaques and lymphocytic choriomeningitis virus infections in mice, the magnitude and timing of the establishment of an excess of effector cells versus targets were found to correlate with the extent of control and the infection outcome (i.e., control and clearance versus partial or poor control and persistent infection). This method highlights the importance of the location, timing, and magnitude of the immune response needed for a vaccine to be effective against agents of persistent infection, such as HIV-1.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

