Expression analysis of the Theileria parva subtelomere-encoded variable secreted protein gene family
- PMID: 19325907
- PMCID: PMC2657828
- DOI: 10.1371/journal.pone.0004839
Expression analysis of the Theileria parva subtelomere-encoded variable secreted protein gene family
Abstract
Background: The intracellular protozoan parasite Theileria parva transforms bovine lymphocytes inducing uncontrolled proliferation. Proteins released from the parasite are assumed to contribute to phenotypic changes of the host cell and parasite persistence. With 85 members, genes encoding subtelomeric variable secreted proteins (SVSPs) form the largest gene family in T. parva. The majority of SVSPs contain predicted signal peptides, suggesting secretion into the host cell cytoplasm.
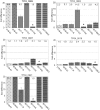
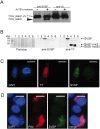
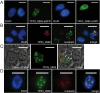
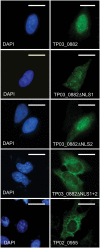
Methodology/principal findings: We analysed SVSP expression in T. parva-transformed cell lines established in vitro by infection of T or B lymphocytes with cloned T. parva parasites. Microarray and quantitative real-time PCR analysis revealed mRNA expression for a wide range of SVSP genes. The pattern of mRNA expression was largely defined by the parasite genotype and not by host background or cell type, and found to be relatively stable in vitro over a period of two months. Interestingly, immunofluorescence analysis carried out on cell lines established from a cloned parasite showed that expression of a single SVSP encoded by TP03_0882 is limited to only a small percentage of parasites. Epitope-tagged TP03_0882 expressed in mammalian cells was found to translocate into the nucleus, a process that could be attributed to two different nuclear localisation signals.
Conclusions: Our analysis reveals a complex pattern of Theileria SVSP mRNA expression, which depends on the parasite genotype. Whereas in cell lines established from a cloned parasite transcripts can be found corresponding to a wide range of SVSP genes, only a minority of parasites appear to express a particular SVSP protein. The fact that a number of SVSPs contain functional nuclear localisation signals suggests that proteins released from the parasite could contribute to phenotypic changes of the host cell. This initial characterisation will facilitate future studies on the regulation of SVSP gene expression and the potential biological role of these enigmatic proteins.
Conflict of interest statement
Figures


References
-
- Barry JD, Ginger ML, Burton P, McCulloch R. Why are parasite contingency genes often associated with telomeres? International Journal for Parasitology. 2003;33:29–45. - PubMed
-
- Borst P, Ulbert S. Control of VSG gene expression sites. Mol Biochem Parasitol. 2001;114:17–27. - PubMed
-
- Filler SG. Candida-host cell receptor-ligand interactions. Curr Opin Microbiol. 2006;9:333–339. - PubMed
-
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

