Cysteine redox potential determines pro-inflammatory IL-1beta levels
- PMID: 19325908
- PMCID: PMC2657829
- DOI: 10.1371/journal.pone.0005017
Cysteine redox potential determines pro-inflammatory IL-1beta levels
Abstract
Background: Cysteine (Cys) and its disulfide, cystine (CySS) represent the major extracellular thiol/disulfide redox control system. The redox potential (E(h)) of Cys/CySS is centered at approximately -80 mV in the plasma of healthy adults, and oxidation of E(h) Cys/CySS is implicated in inflammation associated with various diseases.
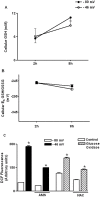
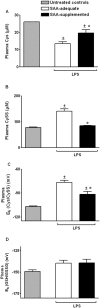
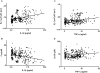
Methodology/principal findings: The purpose of the present study was to determine whether oxidized E(h) Cys/CySS is a determinant of interleukin (IL)-1beta levels. Results showed a 1.7-fold increase in secreted pro-IL-1beta levels in U937 monocytes exposed to oxidized E(h) Cys/CySS (-46 mV), compared to controls exposed to a physiological E(h) of -80 mV (P<0.01). In LPS-challenged mice, preservation of plasma E(h) Cys/CySS from oxidation by dietary sulfur amino acid (SAA) supplementation, was associated with a 1.6-fold decrease in plasma IL-1beta compared to control mice fed an isonitrogenous SAA-adequate diet (P<0.01). Analysis of E(h) Cys/CySS and IL-1beta in human plasma revealed a significant positive association between oxidized E(h) Cys/CySS and IL-1beta after controlling for age, gender, and BMI (P<0.001).
Conclusions/significance: These data show that oxidized extracellular E(h) Cys/CySS is a determinant of IL-1beta levels, and suggest that strategies to preserve E(h) Cys/CySS may represent a means to control IL-1beta in inflammatory disease states.
Conflict of interest statement
Figures


References
-
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–113. - PubMed
-
- Waehre T, Yndestad A, Smith C, Haug T, Tunheim SH, et al. Increased expression of interleukin-1 in coronary artery disease with downregulatory effects of HMG-CoA reductase inhibitors. Circulation. 2004;109:1966–1972. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

