Synergism of heat shock protein 90 and histone deacetylase inhibitors in synovial sarcoma
- PMID: 19325926
- PMCID: PMC2659882
- DOI: 10.1155/2009/794901
Synergism of heat shock protein 90 and histone deacetylase inhibitors in synovial sarcoma
Abstract
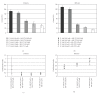
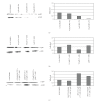
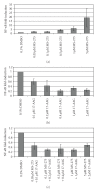
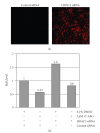
Current systemic therapies have little curative benefit for synovial sarcoma. Histone deacetylase (HDAC) inhibitors and the heat shock protein 90 (Hsp90) inhibitor 17-AAG have recently been shown to inhibit synovial sarcoma in preclinical models. We tested combinations of 17-AAG with the HDAC inhibitor MS-275 for synergism by proliferation and apoptosis assays. The combination was found to be synergistic at multiple time points in two synovial sarcoma cell lines. Previous studies have shown that HDAC inhibitors not only induce cell death but also activate the survival pathway NF-kappaB, potentially limiting therapeutic benefit. As 17-AAG inhibits activators of NF-kappaB, we tested if 17-AAG synergizes with MS-275 through abrogating NF-kappaB activation. In our assays, adding 17-AAG blocks NF-kappaB activation by MS-275 and siRNA directed against histone deacetylase 3 (HDAC3) recapitulates the effects of MS-275. Additionally, we find that the NF-kappaB inhibitor BAY 11-7085 synergizes with MS-275. We conclude that agents inhibiting NF-kappaB synergize with HDAC inhibitors against synovial sarcoma.
Figures

References
-
- Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20(40):5755–5762. - PubMed
-
- Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. - PubMed
-
- Guillou L, Benhattar J, Bonichon F, et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. Journal of Clinical Oncology. 2004;22(20):4040–4050. - PubMed
-
- Takenaka S, Ueda T, Naka N, et al. Prognostic implication of SYT-SSX fusion type in synovial sarcoma: a multi-institutional retrospective analysis in Japan. Oncology Reports. 2008;19(2):467–476. - PubMed
-
- Guadagnolo B, Zagars G, Ballo M, et al. Long-term outcomes for synovial sarcoma treated with conservation surgery and radiotherapy. International Journal of Radiation Oncology, Biology, Physics. 2007;69(4):1173–1180. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

