Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells
- PMID: 19327009
- PMCID: PMC3032563
- DOI: 10.1089/scd.2008.0310
Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells
Abstract
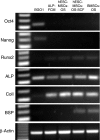
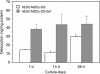
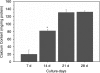
To enhance the understanding of differentiation patterns and bone formation capacity of hESCs, we determined (1) the temporal pattern of osteoblastic differentiation of human embryonic stem cell-derived mesenchymal stem cells (hESC-MSCs), (2) the influence of a three-dimensional matrix on the osteogenic differentiation of hESC-MSCs in long-term culture, and (3) the bone-forming capacity of osteoblast-like cells derived from hESC-MSCs in calvarial defects. Incubation of hESC-MSCs in osteogenic medium induced osteoblastic differentiation of hESC-MSCs into mature osteoblasts in a similar chronological pattern to human bone marrow stromal cells and primary osteoblasts. Osteogenic differentiation was enhanced by culturing the cells on three-dimensional collagen scaffolds. Fluorescent-activated cell sorting of alkaline phosphatase expressing cells was used to obtain an enriched osteogenic cell population for in vivo transplantation. The identification of green fluorescence protein and expression of human-specific nuclear antigen in osteocytes in newly formed bone verified the role of transplanted human cells in the bone regeneration process. The current cell culture model and osteogenic cell enrichment method could provide large numbers of osteoprogenitor cells for analysis of differentiation patterns and cell transplantation to regenerate skeletal defects.
Figures







References
-
- Mimeault M. Hauke R. Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. - PubMed
-
- Kwan MD. Slater BJ. Wan DC. Longaker MT. Cell-based therapies for skeletal regenerative medicine. Hum Mol Genet. 2008;17:R93–R98. - PubMed
-
- Carpenter MK. Rosler E. Rao MS. Characterization and differentiation of human embryonic stem cells. Cloning Stem Cells. 2003;5:79–88. - PubMed
-
- Fenno LE. Ptaszek LM. Cowan CA. Human embryonic stem cells: emerging technologies and practical applications. Curr Opin Genet Dev. 2008;18:324–329. - PubMed
-
- Olivier EN. Rybicki AC. Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

