Investigations into small molecule non-peptidic inhibitors of the botulinum neurotoxins
- PMID: 19327377
- PMCID: PMC2730986
- DOI: 10.1016/j.toxicon.2009.03.016
Investigations into small molecule non-peptidic inhibitors of the botulinum neurotoxins
Abstract
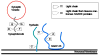
Botulinum neurotoxins (BoNTs), proteins secreted by the bacteria genus Clostridium, represent a group of extremely lethal toxins and a potential bioterrorism threat. As the current therapeutic options are of a predominantly prophylactic nature and cannot be used en masse, new strategies and ultimately potential treatments are desperately needed to combat any widespread release of these neurotoxins. In these regards, our laboratory has been working on developing new alternatives to treat botulinum intoxication through the development of inhibitors of the light chain proteases, the etiological agent which causes BoNT intoxication. Such a strategy has required the construction of two high-throughput screens and small molecule non-peptidic libraries; excitingly, inhibitors of the BoNT/A protease have been uncovered and are being optimized via structure activity relationship studies.
Figures



Similar articles
-
Challenges in searching for therapeutics against Botulinum Neurotoxins.Expert Opin Drug Discov. 2017 May;12(5):497-510. doi: 10.1080/17460441.2017.1303476. Epub 2017 Mar 17. Expert Opin Drug Discov. 2017. PMID: 28271909 Review.
-
Recent developments with metalloprotease inhibitor class of drug candidates for botulinum neurotoxins.Curr Top Med Chem. 2015;15(7):685-95. doi: 10.2174/1568026615666150309150338. Curr Top Med Chem. 2015. PMID: 25751268 Review.
-
Strategies to design inhibitors of Clostridium botulinum neurotoxins.Infect Disord Drug Targets. 2007 Mar;7(1):47-57. doi: 10.2174/187152607780090667. Infect Disord Drug Targets. 2007. PMID: 17346211 Review.
-
Novel small molecule inhibitors of botulinum neurotoxin A metalloprotease activity.Biochem Biophys Res Commun. 2003 Oct 10;310(1):84-93. doi: 10.1016/j.bbrc.2003.08.112. Biochem Biophys Res Commun. 2003. PMID: 14511652
-
Therapeutic efficacy of equine botulism heptavalent antitoxin against all seven botulinum neurotoxins in symptomatic guinea pigs.PLoS One. 2019 Sep 17;14(9):e0222670. doi: 10.1371/journal.pone.0222670. eCollection 2019. PLoS One. 2019. PMID: 31527885 Free PMC article.
Cited by
-
Analysis of Botulinum Neurotoxin Serotype A Metalloprotease Inhibitors: Analogs of a Chemotype for Therapeutic Development in the Context of a Three-Zone Pharmacophore.Open Access Bioinformatics. 2010 Apr 1;2010(2):11-18. doi: 10.2147/OAB.S7251. Open Access Bioinformatics. 2010. PMID: 21103387 Free PMC article.
-
Chirality holds the key for potent inhibition of the botulinum neurotoxin serotype a protease.Org Lett. 2010 Feb 19;12(4):756-9. doi: 10.1021/ol902820z. Org Lett. 2010. PMID: 20092262 Free PMC article.
-
3-Hydroxy-1-alkyl-2-methylpyridine-4(1H)-thiones: Inhibition of the Pseudomonas aeruginosa Virulence Factor LasB.ACS Med Chem Lett. 2012 Aug 9;3(8):668-672. doi: 10.1021/ml300128f. Epub 2012 Jun 19. ACS Med Chem Lett. 2012. PMID: 23181168 Free PMC article.
-
Discovery of Dipeptides as Potent Botulinum Neurotoxin A Light-Chain Inhibitors.ACS Med Chem Lett. 2021 Jan 27;12(2):295-301. doi: 10.1021/acsmedchemlett.0c00674. eCollection 2021 Feb 11. ACS Med Chem Lett. 2021. PMID: 33603978 Free PMC article.
-
Formulating a new basis for the treatment against botulinum neurotoxin intoxication: 3,4-Diaminopyridine prodrug design and characterization.Bioorg Med Chem. 2011 Nov 1;19(21):6203-9. doi: 10.1016/j.bmc.2011.09.019. Epub 2011 Sep 14. Bioorg Med Chem. 2011. PMID: 21975066 Free PMC article.
References
-
- Adler M, Nicholson JD, Hackley BE. Efficacy of a novel metalloprotease inhibitor on botulinum neurotoxin B activity. FEBS Lett. 1998;429 (3):234–238. - PubMed
-
- Anne C, Cornille F, Lenoir C, Roques BP. High-throughput fluorogenic assay for determination of botulinum type B neurotoxin protease activity. Anal Biochem. 2001;291 (2):253–261. - PubMed
-
- Anne C, Turcaud S, Quancard J, Teffo F, Meudal H, Fournie-Zaluski MC, Roques BP. Development of potent inhibitors of botulinum neurotoxin type B. J Med Chem. 2003a;46(22):4648–4656. - PubMed
-
- Anne C, Blommaert A, Turcaud S, Martin A-S, Meudal H, Roques BP. Thio-derived disulfides as potent inhibitors of botulinum neurotoxin type B: implications for zinc interaction. Bioorg Med Chem. 2003b;11(21):4655–4660. - PubMed
-
- Arnon SS, Schechter R, Inglesby TH, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285(8):1059–1079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

