Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial
- PMID: 19327618
- PMCID: PMC2752875
- DOI: 10.1016/j.cardfail.2009.01.013
Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial
Abstract
Background: SERCA2a deficiency is commonly seen in advanced heart failure (HF). This study is designed to investigate safety and biological effects of enzyme replacement using gene transfer in patients with advanced HF.
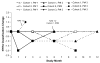
Methods and results: A total of 9 patients with advanced HF (New York Heart Association [NYHA] Class III/IV, ejection fraction [EF] < or = 30%, maximal oxygen uptake [VO2 max] <16 mL.kg.min, with maximal pharmacological and device therapy) received a single intracoronary infusion of AAV1/SERCA2a in the open-label portion of this ongoing study. Doses administered ranged from 1.4 x 10(11) to 3 x 10(12) DNase resistant particles per patient. We present 6- to 12-month follow-up data for these patients. AAV1/SERCA2a demonstrated an acceptable safety profile in this advanced HF population. Of the 9 patients treated, several demonstrated improvements from baseline to month 6 across a number of parameters important in HF, including symptomatic (NYHA and Minnesota Living with Heart Failure Questionnaire, 5 patients), functional (6-minute walk test and VO2 max, 4 patients), biomarker (NT-ProBNP, 2 patients), and LV function/remodeling (EF and end-systolic volume, 5 patients). Of note, 2 patients who failed to improve had preexisting anti-AAV1 neutralizing antibodies.
Conclusions: Quantitative evidence of biological activity across a number of parameters important for assessing HF status could be detected in several patients without preexisting neutralizing antibodies in this open-label study, although the number of patients in each cohort is too small to conduct statistical analyses. These findings support the initiation of the Phase 2 double-blind, placebo-controlled portion of this study.
Figures


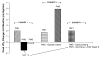



References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson T, Flegal K, et al. Heart Disease and stroke statistics–2009 update. A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation Epub. 2008 Dec 15;119:e21–181. - PubMed
-
- Cleland J, Swedberg K, Poole-Wilson P. Successes and failures of current treatment of heart failure. Lancet. 1998;352(Suppl 1):SI19–28. - PubMed
-
- Goldstein D, Oz M, Rose E. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–33. - PubMed
-
- Hajjar R, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–67. - PubMed
-
- del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001;88:E66–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

