Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2
- PMID: 19327734
- PMCID: PMC2667973
- DOI: 10.1016/j.ajhg.2009.03.001
Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2
Abstract
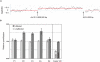
Autosomal-dominant brachydactyly type A2 (BDA2), a limb malformation characterized by hypoplastic middle phalanges of the second and fifth fingers, has been shown to be due to mutations in the Bone morphogenetic protein receptor 1B (BMPR1B) or in its ligand Growth and differentiation factor 5 (GDF5). A linkage analysis performed in a mutation-negative family identified a novel locus for BDA2 on chromosome 20p12.3 that incorporates the gene for Bone morphogenetic protein 2 (BMP2). No point mutation was identified in BMP2, so a high-density array CGH analysis covering the critical interval of approximately 1.3 Mb was performed. A microduplication of approximately 5.5 kb in a noncoding sequence approximately 110 kb downstream of BMP2 was detected. Screening of other patients by qPCR revealed a similar duplication in a second family. The duplicated region contains evolutionary highly conserved sequences suggestive of a long-range regulator. By using a transgenic mouse model we can show that this sequence is able to drive expression of a X-Gal reporter construct in the limbs. The almost complete overlap with endogenous Bmp2 expression indicates that a limb-specific enhancer of Bmp2 is located within the identified duplication. Our results reveal an additional functional mechanism for the pathogenesis of BDA2, which is duplication of a regulatory element that affects the expression of BMP2 in the developing limb.
Figures


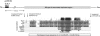

Comment in
-
Non-coding regulatory genetics of limb malformations.Clin Genet. 2009 Dec;76(6):499-501. doi: 10.1111/j.1399-0004.2009.01296.x. Clin Genet. 2009. PMID: 19930151 No abstract available.
References
-
- Howard M.L., Davidson E.H. cis-Regulatory control circuits in development. Dev. Biol. 2004;271:109–118. - PubMed
-
- Kishigami S., Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. - PubMed
-
- Seemann P., Mundlos S., Lehmann K. Alterations of bone morphogenetic protein signaling pathway(s) in skeletal diseases. In: Vukicevic S., Sampath K.T., editors. Bone Morphogenetic Proteins: From Local to Systemic Therapeutics. Birkhäuser Verlag AG; Basel: 2008. pp. 141–159.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

