Methamphetamine toxicity and messengers of death
- PMID: 19328213
- PMCID: PMC2731235
- DOI: 10.1016/j.brainresrev.2009.03.002
Methamphetamine toxicity and messengers of death
Abstract
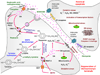
Methamphetamine (METH) is an illicit psychostimulant that is widely abused in the world. Several lines of evidence suggest that chronic METH abuse leads to neurodegenerative changes in the human brain. These include damage to dopamine and serotonin axons, loss of gray matter accompanied by hypertrophy of the white matter and microgliosis in different brain areas. In the present review, we summarize data on the animal models of METH neurotoxicity which include degeneration of monoaminergic terminals and neuronal apoptosis. In addition, we discuss molecular and cellular bases of METH-induced neuropathologies. The accumulated evidence indicates that multiple events, including oxidative stress, excitotoxicity, hyperthermia, neuroinflammatory responses, mitochondrial dysfunction, and endoplasmic reticulum stress converge to mediate METH-induced terminal degeneration and neuronal apoptosis. When taken together, these findings suggest that pharmacological strategies geared towards the prevention and treatment of the deleterious effects of this drug will need to attack the various pathways that form the substrates of METH toxicity.
Figures
References
-
- Abekawa T, Ohmori T, Koyama T. Effects of repeated administration of a high dose of methamphetamine on dopamine and glutamate release in rat striatum and nucleus accumbens. Brain Res. 1994;643:276–281. - PubMed
-
- Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;30:1128–1137. - PubMed
-
- Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;32:531–541. - PubMed
-
- Acikgoz O, Gonenc S, Kayatekin BM, Uysal N, Pekcetin C, Semin I, Gure A. Methamphetamine causes lipid peroxidation and an increase in superoxide dismutase activity in the rat striatum. Brain Res. 1998;813:200–202. - PubMed
-
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J. Pharmacol. Exp. Ther. 1995;275:1104–1114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

