Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA)
- PMID: 19329436
- PMCID: PMC2685660
- DOI: 10.1074/jbc.M808752200
Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA)
Abstract
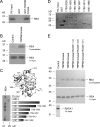
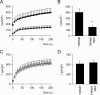
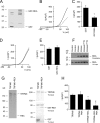
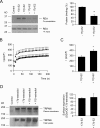
The maintenance of the Mg(2+) balance of the body is essential for neuromuscular excitability, protein synthesis, nucleic acid stability, and numerous enzymatic systems. The Transient Receptor Potential Melastatin 6 (TRPM6) functions as the gatekeeper of transepithelial Mg(2+) transport. However, the molecular regulation of TRPM6 channel activity remains elusive. Here, we identified the repressor of estrogen receptor activity (REA) as an interacting protein of TRPM6 that binds to the 6(th), 7(th), and 8(th) beta-sheets in its alpha-kinase domain. Importantly, REA and TRPM6 are coexpressed in renal Mg(2+)-transporting distal convoluted tubules (DCT). We demonstrated that REA significantly inhibits TRPM6, but not its closest homologue TRPM7, channel activity. This inhibition occurs in a phosphorylation-dependent manner, since REA has no effect on the TRPM6 phosphotransferase-deficient mutant (K1804R), while it still binds to this mutant. Moreover, activation of protein kinase C by phorbol 12-myristate 13-acetate-PMA potentiated the inhibitory effect of REA on TRPM6 channel activity. Finally, we showed that the interaction between REA and TRPM6 is a dynamic process, as short-term 17beta-estradiol treatment disassociates the binding between these proteins. In agreement with this, 17beta-estradiol treatment significantly stimulates the TRPM6-mediated current in HEK293 cells. These results suggest a rapid pathway for the effect of estrogen on Mg(2+) homeostasis in addition to its transcriptional effect. Together, these data indicate that REA operates as a negative feedback modulator of TRPM6 in the regulation of active Mg(2+) (re)absorption and provides new insight into the molecular mechanism of renal transepithelial Mg(2+) transport.
Figures

Similar articles
-
RACK1 inhibits TRPM6 activity via phosphorylation of the fused alpha-kinase domain.Curr Biol. 2008 Feb 12;18(3):168-76. doi: 10.1016/j.cub.2007.12.058. Curr Biol. 2008. PMID: 18258429
-
Epithelial Mg2+ channel TRPM6: insight into the molecular regulation.Magnes Res. 2009 Sep;22(3):127-32. Magnes Res. 2009. PMID: 19780399 Review.
-
Role of the alpha-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP.J Biol Chem. 2008 Jul 18;283(29):19999-20007. doi: 10.1074/jbc.M800167200. Epub 2008 May 19. J Biol Chem. 2008. PMID: 18490453
-
The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens.J Am Soc Nephrol. 2006 Apr;17(4):1035-43. doi: 10.1681/ASN.2005070700. Epub 2006 Mar 8. J Am Soc Nephrol. 2006. PMID: 16524949
-
TRPM6: A Janus-like protein.Handb Exp Pharmacol. 2007;(179):299-311. doi: 10.1007/978-3-540-34891-7_18. Handb Exp Pharmacol. 2007. PMID: 17217065 Review.
Cited by
-
Distal convoluted tubule.Compr Physiol. 2015 Jan;5(1):45-98. doi: 10.1002/cphy.c140002. Compr Physiol. 2015. PMID: 25589264 Free PMC article. Review.
-
Mice defective in Trpm6 show embryonic mortality and neural tube defects.Hum Mol Genet. 2009 Nov 15;18(22):4367-75. doi: 10.1093/hmg/ddp392. Epub 2009 Aug 18. Hum Mol Genet. 2009. PMID: 19692351 Free PMC article.
-
Role of renal TRP channels in physiology and pathology.Semin Immunopathol. 2016 May;38(3):371-83. doi: 10.1007/s00281-015-0527-z. Epub 2015 Sep 18. Semin Immunopathol. 2016. PMID: 26385481 Free PMC article. Review.
-
The alpha-kinase family: an exceptional branch on the protein kinase tree.Cell Mol Life Sci. 2010 Mar;67(6):875-90. doi: 10.1007/s00018-009-0215-z. Epub 2009 Dec 12. Cell Mol Life Sci. 2010. PMID: 20012461 Free PMC article. Review.
-
Regulation of Mg2+ Reabsorption and Transient Receptor Potential Melastatin Type 6 Activity by cAMP Signaling.J Am Soc Nephrol. 2016 Mar;27(3):804-13. doi: 10.1681/ASN.2014121228. Epub 2015 Jul 6. J Am Soc Nephrol. 2016. PMID: 26150606 Free PMC article.
References
-
- Vetter, T., and Lohse, M. J. (2002) Curr. Opin. Nephrol. Hypertens. 11 403–410 - PubMed
-
- Grubbs, R. D. (2002) Biometals 15 251–259 - PubMed
-
- Romani, A., and Scarpa, A. (1992) Arch. Biochem. Biophys. 298 1–12 - PubMed
-
- Konrad, M., Schlingmann, K. P., and Gudermann, T. (2004) Am. J. Physiol. Renal Physiol. 286 599–605 - PubMed
-
- Chubanov, V., Gudermann, T., and Schlingmann, K. P. (2005) Pflugers Arch. 451 228–234 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

