Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis
- PMID: 19329558
- PMCID: PMC2671706
- DOI: 10.1105/tpc.108.063164
Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis
Abstract
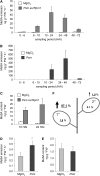
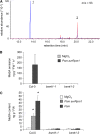
Systemic acquired resistance (SAR) develops in response to local microbial leaf inoculation and renders the whole plant more resistant to subsequent pathogen infection. Accumulation of salicylic acid (SA) in noninfected plant parts is required for SAR, and methyl salicylate (MeSA) and jasmonate (JA) are proposed to have critical roles during SAR long-distance signaling from inoculated to distant leaves. Here, we address the significance of MeSA and JA during SAR development in Arabidopsis thaliana. MeSA production increases in leaves inoculated with the SAR-inducing bacterial pathogen Pseudomonas syringae; however, most MeSA is emitted into the atmosphere, and only small amounts are retained. We show that in several Arabidopsis defense mutants, the abilities to produce MeSA and to establish SAR do not coincide. T-DNA insertion lines defective in expression of a pathogen-responsive SA methyltransferase gene are completely devoid of induced MeSA production but increase systemic SA levels and develop SAR upon local P. syringae inoculation. Therefore, MeSA is dispensable for SAR in Arabidopsis, and SA accumulation in distant leaves appears to occur by de novo synthesis via isochorismate synthase. We show that MeSA production induced by P. syringae depends on the JA pathway but that JA biosynthesis or downstream signaling is not required for SAR. In compatible interactions, MeSA production depends on the P. syringae virulence factor coronatine, suggesting that the phytopathogen uses coronatine-mediated volatilization of MeSA from leaves to attenuate the SA-based defense pathway.
Figures






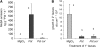
References
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Ament, K., Van Schie, C.C., Bouwmeester, H.J., Haring, M.A., and Schuurink, R.C. (2006). Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta 224 1197–1208. - PubMed
-
- Attaran, E., Rostás, M., and Zeier, J. (2008). Pseudomonas syringae elicits emission of the terpenoid (E,E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene in Arabidopsis leaves via jasmonate signaling and expression of the terpene synthase TPS4. Mol. Plant Microbe Interact. 21 1482–1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

