The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination
- PMID: 19329562
- PMCID: PMC2675718
- DOI: 10.1104/pp.109.138107
The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination
Abstract
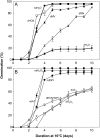
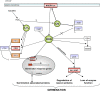
The physiological dormancy of sunflower (Helianthus annuus) embryos can be overcome during dry storage (after-ripening) or by applying exogenous ethylene or hydrogen cyanide (HCN) during imbibition. The aim of this work was to provide a comprehensive model, based on oxidative signaling by reactive oxygen species (ROS), for explaining the cellular mode of action of HCN in dormancy alleviation. Beneficial HCN effect on germination of dormant embryos is associated with a marked increase in hydrogen peroxide and superoxide anion generation in the embryonic axes. It is mimicked by the ROS-generating compounds methylviologen and menadione but suppressed by ROS scavengers. This increase results from an inhibition of catalase and superoxide dismutase activities and also involves activation of NADPH oxidase. However, it is not related to lipid reserve degradation or gluconeogenesis and not associated with marked changes in the cellular redox status controlled by the glutathione/glutathione disulfide couple. The expression of genes related to ROS production (NADPHox, POX, AO1, and AO2) and signaling (MAPK6, Ser/ThrPK, CaM, and PTP) is differentially affected by dormancy alleviation either during after-ripening or by HCN treatment, and the effect of cyanide on gene expression is likely to be mediated by ROS. It is also demonstrated that HCN and ROS both activate similarly ERF1, a component of the ethylene signaling pathway. We propose that ROS play a key role in the control of sunflower seed germination and are second messengers of cyanide in seed dormancy release.
Figures


References
-
- Amory AM, Ford L, Pammenter NW, Cresswell CF (1992) The use of 3-amino-1,2,4-triazole to investigate the short-term effects of oxygen toxicity on carbon assimilation by Pisum sativum seedlings. Plant Cell Environ 15 655–663
-
- Bailly C, Benamar A, Corbineau F, Côme D (1996) Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol Plant 97 104–110
-
- Bailly C, Bogatek-Leszczynska R, Côme D, Corbineau F (2002) Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci Res 12 47–55
-
- Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331 806–814 - PubMed
-
- Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease and oxidative stress. J Biol Chem 272 20313–20316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

