Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos
- PMID: 19329563
- PMCID: PMC2675710
- DOI: 10.1104/pp.109.137737
Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos
Abstract
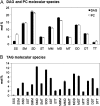
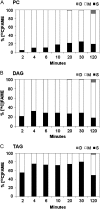
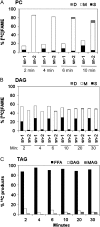
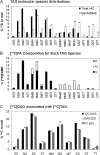
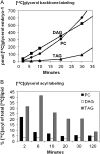
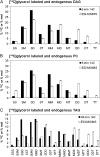
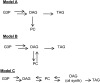
The reactions leading to triacylglycerol (TAG) synthesis in oilseeds have been well characterized. However, quantitative analyses of acyl group and glycerol backbone fluxes that comprise extraplastidic phospholipid and TAG synthesis, including acyl editing and phosphatidylcholine-diacylglycerol interconversion, are lacking. To investigate these fluxes, we rapidly labeled developing soybean (Glycine max) embryos with [(14)C]acetate and [(14)C]glycerol. Cultured intact embryos that mimic in planta growth were used. The initial kinetics of newly synthesized acyl chain and glycerol backbone incorporation into phosphatidylcholine (PC), 1,2-sn-diacylglycerol (DAG), and TAG were analyzed along with their initial labeled molecular species and positional distributions. Almost 60% of the newly synthesized fatty acids first enter glycerolipids through PC acyl editing, largely at the sn-2 position. This flux, mostly of oleate, was over three times the flux of nascent [(14)C]fatty acids incorporated into the sn-1 and sn-2 positions of DAG through glycerol-3-phosphate acylation. Furthermore, the total flux for PC acyl editing, which includes both nascent and preexisting fatty acids, was estimated to be 1.5 to 5 times the flux of fatty acid synthesis. Thus, recycled acyl groups (16:0, 18:1, 18:2, and 18:3) in the acyl-coenzyme A pool provide most of the acyl chains for de novo glycerol-3-phosphate acylation. Our results also show kinetically distinct DAG pools. DAG used for TAG synthesis is mostly derived from PC, whereas de novo synthesized DAG is mostly used for PC synthesis. In addition, two kinetically distinct sn-3 acylations of DAG were observed, providing TAG molecular species enriched in saturated or polyunsaturated fatty acids.
Figures



References
-
- Allen DK, Ohlrogge JB, Shachar-Hill Y (2009) The role of light in soybean seed filling metabolism. Plant J 58 220–234 - PubMed
-
- Allen DK, Shachar-Hill Y, Ohlrogge JB (2007) Compartment-specific labeling information in C-13 metabolic flux analysis of plants. Phytochemistry 68 2197–2210 - PubMed
-
- Bates PD, Ohlrogge JB, Pollard M (2007) Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem 282 31206–31216 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

