A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi
- PMID: 19329566
- PMCID: PMC2675747
- DOI: 10.1104/pp.109.136390
A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi
Abstract
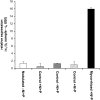
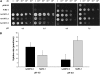
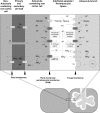
In mycorrhizal associations, the fungal partner assists its plant host by providing nitrogen (N) in addition to phosphate. Arbuscular mycorrhizal (AM) fungi have access to inorganic or organic forms of N and translocate them via arginine from the extra- to the intraradical mycelium, where the N is transferred to the plant without any carbon skeleton. However, the molecular form in which N is transferred, as well as the involved mechanisms, is still under debate. NH(4)(+) seems to be the preferential transferred molecule, but no plant ammonium transporter (AMT) has been identified so far. Here, we offer evidence of a plant AMT that is involved in N uptake during mycorrhiza symbiosis. The gene LjAMT2;2, which has been shown to be the highest up-regulated gene in a transcriptomic analysis of Lotus japonicus roots upon colonization with Gigaspora margarita, has been characterized as a high-affinity AMT belonging to the AMT2 subfamily. It is exclusively expressed in the mycorrhizal roots, but not in the nodules, and transcripts have preferentially been located in the arbusculated cells. Yeast (Saccharomyces cerevisiae) mutant complementation has confirmed its functionality and revealed its dependency on acidic pH. The transport experiments using Xenopus laevis oocytes indicated that, unlike other plant AMTs, LjAMT2;2 transports NH(3) instead of NH(4)(+). Our results suggest that the transporter binds charged ammonium in the apoplastic interfacial compartment and releases the uncharged NH(3) into the plant cytoplasm. The implications of such a finding are discussed in the context of AM functioning and plant phosphorus uptake.
Figures





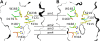
Similar articles
-
LjAMT2;2 Promotes Ammonium Nitrogen Transport during Arbuscular Mycorrhizal Fungi Symbiosis in Lotus japonicus.Int J Mol Sci. 2022 Aug 23;23(17):9522. doi: 10.3390/ijms23179522. Int J Mol Sci. 2022. PMID: 36076919 Free PMC article.
-
Transcriptional regulation of host NH₄⁺ transporters and GS/GOGAT pathway in arbuscular mycorrhizal rice roots.Plant Physiol Biochem. 2014 Feb;75:1-8. doi: 10.1016/j.plaphy.2013.11.029. Epub 2013 Dec 10. Plant Physiol Biochem. 2014. PMID: 24361504
-
Functional Characterization of Ammonium Transporter LjAMT2;4 During Lotus japonicus Symbiosis with Rhizobia and Arbuscular Mycorrhizal Fungi.J Fungi (Basel). 2025 Apr 27;11(5):340. doi: 10.3390/jof11050340. J Fungi (Basel). 2025. PMID: 40422674 Free PMC article.
-
[Metabolism and interaction of C and N in the arbuscular mycorrhizal symbiosis].Ying Yong Sheng Tai Xue Bao. 2014 Mar;25(3):903-10. Ying Yong Sheng Tai Xue Bao. 2014. PMID: 24984513 Review. Chinese.
-
Forms of nitrogen uptake, translocation, and transfer via arbuscular mycorrhizal fungi: a review.Sci China Life Sci. 2012 Jun;55(6):474-82. doi: 10.1007/s11427-012-4330-y. Epub 2012 Jun 29. Sci China Life Sci. 2012. PMID: 22744177 Review.
Cited by
-
Kinetics of NH (4) (+) uptake by the arbuscular mycorrhizal fungus Rhizophagus irregularis.Mycorrhiza. 2012 Aug;22(6):485-91. doi: 10.1007/s00572-012-0452-0. Epub 2012 Jul 1. Mycorrhiza. 2012. PMID: 22752460
-
Gender-related responses of dioecious plant Populus cathayana to AMF, drought and planting pattern.Sci Rep. 2020 Jul 13;10(1):11530. doi: 10.1038/s41598-020-68112-0. Sci Rep. 2020. PMID: 32661316 Free PMC article.
-
Evidence that arbuscular mycorrhizal and phosphate-solubilizing fungi alleviate NaCl stress in the halophyte Kosteletzkya virginica: nutrient uptake and ion distribution within root tissues.Mycorrhiza. 2014 Jul;24(5):383-95. doi: 10.1007/s00572-013-0546-3. Epub 2013 Dec 17. Mycorrhiza. 2014. PMID: 24343115
-
Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots.Sci Rep. 2018 Jan 8;8(1):4. doi: 10.1038/s41598-017-18445-0. Sci Rep. 2018. PMID: 29311719 Free PMC article.
-
Functional Characterization of the Arabidopsis Ammonium Transporter AtAMT1;3 With the Emphasis on Structural Determinants of Substrate Binding and Permeation Properties.Front Plant Sci. 2020 May 21;11:571. doi: 10.3389/fpls.2020.00571. eCollection 2020. Front Plant Sci. 2020. PMID: 32528489 Free PMC article.
References
-
- Balestrini R, Gomez-Ariza J, Lanfranco L, Bonfante P (2007) Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol Plant Microbe Interact 20 1055–1062 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

