Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging
- PMID: 19330002
- PMCID: PMC2712647
- DOI: 10.1038/nn.2295
Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging
Abstract
Hair cells detect vibrations of their stereociliary bundle by activation of mechanically sensitive transducer channels. Although evidence suggests the transducer channels are near the stereociliary tops and are opened by force imparted by tip links connecting contiguous stereocilia, the exact channel site remains controversial. We used fast confocal imaging of fluorescence changes reflecting calcium entry during bundle stimulation to localize the channels. Calcium signals were visible in single stereocilia of rat cochlear inner hair cells and were up to tenfold larger and faster in the second and third stereociliary rows than in the tallest first row. The number of functional stereocilia was proportional to transducer current amplitude, indicating that there were about two channels per stereocilium. Comparable results were obtained in outer hair cells. The observations, supported by theoretical simulations, suggest there are no functional mechanically sensitive transducer channels in first row stereocilia and imply the channels are present only at the bottom of the tip links.
Figures
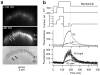
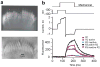
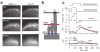
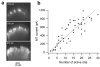


Comment in
-
Bottoms up: transduction channels at tip link bases.Nat Neurosci. 2009 May;12(5):529-30. doi: 10.1038/nn0509-529. Nat Neurosci. 2009. PMID: 19396230 No abstract available.
References
-
- Fettiplace R, Ricci AJ. Mechanoelectrical transduction in auditory hair cells. In: Eatock RA, Popper A, Fay RR, editors. Springer Handbook of Auditory Research: Hair Cells. Springer; Germany: 2006. pp. 154–203.
-
- Jaramillo F, Hudspeth AJ. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron. 1991;7:409–420. - PubMed
-
- Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

