Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature
- PMID: 19330034
- PMCID: PMC2659423
- DOI: 10.1371/journal.pone.0004972
Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature
Abstract
Background: Under the direction and sponsorship of the National Cancer Institute, we report on the first pre-clinical trial of the Comparative Oncology Trials Consortium (COTC). The COTC is a novel infrastructure to integrate cancers that naturally develop in pet dogs into the development path of new human drugs. Trials are designed to address questions challenging in conventional preclinical models and early phase human trials. Large animal spontaneous cancer models can be a valuable addition to successful studies of cancer biology and novel therapeutic drug, imaging and device development.
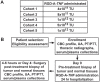
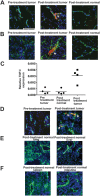
Methodology/principal findings: Through this established infrastructure, the first trial of the COTC (COTC001) evaluated a targeted AAV-phage vector delivering tumor necrosis factor (RGD-A-TNF) to alphaV integrins on tumor endothelium. Trial progress and data was reviewed contemporaneously using a web-enabled electronic reporting system developed for the consortium. Dose-escalation in cohorts of 3 dogs (n = 24) determined an optimal safe dose (5x10(12) transducing units intravenous) of RGD-A-TNF. This demonstrated selective targeting of tumor-associated vasculature and sparing of normal tissues assessed via serial biopsy of both tumor and normal tissue. Repetitive dosing in a cohort of 14 dogs, at the defined optimal dose, was well tolerated and led to objective tumor regression in two dogs (14%), stable disease in six (43%), and disease progression in six (43%) via Response Evaluation Criteria in Solid Tumors (RECIST).
Conclusions/significance: The first study of the COTC has demonstrated the utility and efficiency of the established infrastructure to inform the development of new cancer drugs within large animal naturally occurring cancer models. The preclinical evaluation of RGD-A-TNF within this network provided valuable and necessary data to complete the design of first-in-man studies.
Conflict of interest statement
Figures


References
-
- Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. - PubMed
-
- Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. - PubMed
-
- Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, et al. The dog as a cancer model. Nat Biotechnol. 2006;24:1065–1066. - PubMed
-
- Khanna C, Vail DM. Targeting the lung: preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancers. Curr Cancer Drug Targets. 2003;3:265–273. - PubMed
-
- Larue SM, Fox MH, Ogilvie GK, Page RL, Getzy DM, et al. Tumour cell kinetics as predictors of response in canine lymphoma treated with chemotherapy alone or combined with whole body hyperthermia. Int J Hyperthermia. 1999;15:475–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

