Upregulation of heme oxygenase-1 combined with increased adiponectin lowers blood pressure in diabetic spontaneously hypertensive rats through a reduction in endothelial cell dysfunction, apoptosis and oxidative stress
- PMID: 19330083
- PMCID: PMC2635644
- DOI: 10.3390/ijms9122388
Upregulation of heme oxygenase-1 combined with increased adiponectin lowers blood pressure in diabetic spontaneously hypertensive rats through a reduction in endothelial cell dysfunction, apoptosis and oxidative stress
Abstract
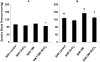
This study was designed to investigate the effect of increased levels of HO-1 on hypertension exacerbated by diabetes. Diabetic spontaneously hypertensive rat (SHR) and WKY (control) animals were treated with streptozotocin (STZ) to induce diabetes and stannous chloride (SnCl(2)) to upregulate HO-1. Treatment with SnCl(2) not only attenuated the increase of blood pressure (p<0.01), but also increased HO-1 protein content, HO activity and plasma adiponectin levels, decreased the levels of superoxide and 3-nitrotyrosine (NT), respectively. Reduction in oxidative stress resulted in the increased expression of Bcl-2 and AKT with a concomitant reduction in circulating endothelial cells (CEC) in the peripheral blood (p<0.005) and an improvement of femoral reactivity (response to acetylcholine). Thus induction of HO-1 accompanied with increased plasma adiponectin levels in diabetic hypertensive rats alters the phenotype through a reduction in oxidative stress, thereby permitting endothelial cells to maintain an anti-apoptotic environment and the restoration of endothelial responses thus preventing hypertension.
Keywords: Heme oxygenase; adiponectin; apoptosis; diabetes; hypertension; oxidative stress.
Figures


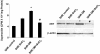

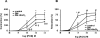



References
-
- Jackowski L, Crockett J, Rowett D. Adults with diabetes - pharmacological management of hypertension. Aust. Fam. Physician. 2008;37:419–421. - PubMed
-
- Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004;279:42351–42354. - PubMed
-
- Bahia L, Aguiar LG, Villela N, Bottino D, Godoy-Matos AF, Geloneze B, Tambascia M, Bouskela E. Relationship between adipokines, inflammation, and vascular reactivity in lean controls and obese subjects with metabolic syndrome. Clinics. 2006;61:433–440. - PubMed
-
- Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

