Dose-response-a challenge for allelopathy?
- PMID: 19330161
- PMCID: PMC2657948
- DOI: 10.2201/nonlin.003.02.002
Dose-response-a challenge for allelopathy?
Abstract
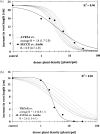
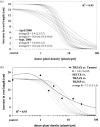
The response of an organism to a chemical depends, among other things, on the dose. Nonlinear dose-response relationships occur across a broad range of research fields, and are a well established tool to describe the basic mechanisms of phytotoxicity. The responses of plants to allelochemicals as biosynthesized phytotoxins, relate as well to nonlinearity and, thus, allelopathic effects can be adequately quantified by nonlinear mathematical modeling. The current paper applies the concept of nonlinearity to assorted aspects of allelopathy within several bioassays and reveals their analysis by nonlinear regression models. Procedures for a valid comparison of effective doses between different allelopathic interactions are presented for both, inhibitory and stimulatory effects. The dose-response applications measure and compare the responses produced by pure allelochemicals [scopoletin (7-hydroxy-6-methoxy-2H-1-benzopyran-2-one); DIBOA (2,4-dihydroxy-2H-1,4-benzoxaxin-3(4H)-one); BOA (benzoxazolin-2(3H)-one); MBOA (6-methoxy-benzoxazolin-2(3H)-one)], involved in allelopathy of grain crops, to demonstrate how some general principles of dose responses also relate to allelopathy. Hereupon, dose-response applications with living donor plants demonstrate the validity of these principles for density-dependent phytotoxicity of allelochemicals produced and released by living plants (Avena sativa L., Secale cereale L., Triticum L. spp.), and reveal the use of such experiments for initial considerations about basic principles of allelopathy. Results confirm that nonlinearity applies to allelopathy, and the study of allelopathic effects in dose-response experiments allows for new and challenging insights into allelopathic interactions.
Keywords: benzoxazinoids; hormesis; log-logistic model; scopoletin.
Figures











References
-
- An M, Johnson IR, Lovett JV. Mathematical modeling of allelopathy: biological response to allelochemicals and its interpretations. J Chem Ecol. 1993;19:2379–2388. - PubMed
-
- An M, Liu DL, Johnson IR, Lovett JV. Mathematical modelling of allelopathy. II. The dynamics of allelochemicals from living plants in the environment. Ecological Modelling. 2003;161:53–66.
-
- Andreae WA. Effects of scopoletin on indoleacetic acid metabolism. Nature. 1952;170:83–84. - PubMed
-
- Baghestani A, Lemieux C, Leroux GD, Baziramakenga R, Simard RR. Determination of allelochemicals in spring cereal cultivars of different competitiveness. Weed Sci. 1999;47:498–504.
-
- Barnes JP, Putnam AR. Role of benzoxazinones in allelopathy of rye (Secale cereale L.) J Chem Ecol. 1987;13:889–906. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

