Diastereoselective pyrrolidine synthesis via copper promoted intramolecular aminooxygenation of alkenes: formal synthesis of (+)-monomorine
- PMID: 19331361
- PMCID: PMC2714983
- DOI: 10.1021/ol9003492
Diastereoselective pyrrolidine synthesis via copper promoted intramolecular aminooxygenation of alkenes: formal synthesis of (+)-monomorine
Abstract
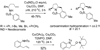
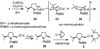
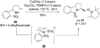
The diastereoselectivity of the copper-promoted intramolecular aminooxygenation of various alkene substrates was investigated. Alpha-substituted 4-pentenyl sulfonamides favor the formation of 2,5-cis-pyrrolidines (dr >20:1) giving excellent yields which range from 76-97% while gamma-substituted substrates favor the 2,3-trans pyrrolidine adducts with moderate selectivity (ca. 3:1). A substrate whose N-substituent was directly tethered to the alpha-carbon exclusively yielded the 2,5-trans pyrrolidine. The synthetic utility of the method was demonstrated by a short and efficient formal synthesis of (+)-monomorine.
Figures

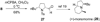
Similar articles
-
General strategy for the construction of enantiopure pyrrolidine-based alkaloids. Total synthesis of (-)-monomorine.J Org Chem. 2007 Apr 13;72(8):3133-6. doi: 10.1021/jo062532p. Epub 2007 Mar 16. J Org Chem. 2007. PMID: 17362042
-
Concise synthesis of pyrrolidine and indolizidine alkaloids by a highly convergent three-component reaction.Chemistry. 2011 Mar 7;17(11):3207-12. doi: 10.1002/chem.201003137. Epub 2011 Feb 9. Chemistry. 2011. PMID: 21308816
-
Total synthesis of (S)-(+)-tylophorine via enantioselective intramolecular alkene carboamination.J Org Chem. 2008 Aug 1;73(15):6045-7. doi: 10.1021/jo801024h. Epub 2008 Jun 28. J Org Chem. 2008. PMID: 18588345 Free PMC article.
-
Palladium(II)-catalyzed enantio- and diastereoselective synthesis of pyrrolidine derivatives.Org Lett. 2012 Aug 17;14(16):4074-7. doi: 10.1021/ol3016989. Epub 2012 Aug 8. Org Lett. 2012. PMID: 22873944 Free PMC article.
-
Chiral amines as organocatalysts for asymmetric conjugate addition to nitroolefins and vinyl sulfones via enamine activation.Chem Commun (Camb). 2007 Aug 14;(30):3123-35. doi: 10.1039/b701216k. Epub 2007 Mar 21. Chem Commun (Camb). 2007. PMID: 17653365 Review.
Cited by
-
Palladium-catalyzed enantioselective addition of two distinct nucleophiles across alkenes capable of quinone methide formation.J Am Chem Soc. 2009 Dec 2;131(47):17074-5. doi: 10.1021/ja909030c. J Am Chem Soc. 2009. PMID: 19902942 Free PMC article.
-
Stereoselective isoxazolidine synthesis via copper-catalyzed alkene aminooxygenation.J Org Chem. 2012 Sep 7;77(17):7755-60. doi: 10.1021/jo3013226. Epub 2012 Aug 15. J Org Chem. 2012. PMID: 22870912 Free PMC article.
-
Iron catalyzed asymmetric oxyamination of olefins.J Am Chem Soc. 2012 Aug 1;134(30):12370-3. doi: 10.1021/ja3046684. Epub 2012 Jul 23. J Am Chem Soc. 2012. PMID: 22793789 Free PMC article.
-
Evidence for alkene cis-aminocupration, an aminooxygenation case study: kinetics, EPR spectroscopy, and DFT calculations.Chemistry. 2012 Feb 6;18(6):1711-26. doi: 10.1002/chem.201101703. Epub 2012 Jan 11. Chemistry. 2012. PMID: 22237868 Free PMC article.
-
Incorporating azaheterocycle functionality in intramolecular aerobic, copper-catalyzed aminooxygenation of alkenes.RSC Adv. 2024 Sep 10;14(39):28822-28826. doi: 10.1039/d4ra06178k. eCollection 2024 Sep 4. RSC Adv. 2024. PMID: 39257658 Free PMC article.
References
-
- Nash RJ, Fellows LE, Dring JV, Fleet GWJ, Derome AE, Hamor TA, Scofield AM, Watkin DJ. Tetrahedron Lett. 1988;29:2487–2490.
- Scofield AM, Rossiter JT, Witham P, Kite GC, Nash RJ, Fellows LE. Phytochemistry. 1990;29:107–109.
-
- Kato A, Kano E, Adachi I, Molyneux RJ, Watson AA, Nash RJ, Fleet GWJ, Wormald MR, Kizu H, Ikeda K, Asano N. Tetrahedron: Asymmetry. 2003;14:325–331.
- Molyneux RJ, Benson M, Wong RY, Tropea JE, Elbein AD. J. Nat. Prod. 1988;51:1198–1206.
-
- Powell RG, Weisleder D, Smith CR, Jr, Rohwedder WK. Tetrahedron Lett. 1970;11:815–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

