N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists
- PMID: 19331412
- PMCID: PMC2760932
- DOI: 10.1021/jm900095y
N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists
Abstract
In the present report, the D3 receptor pharmacophore is modified in the 2,3-diCl- and 2-OCH(3)-phenylpiperazine class of compounds with the goal to improve D3 receptor affinity and selectivity. This extension of structure-activity relationships (SAR) has resulted in the identification of the first enantioselective D3 antagonists (R- and S-22) to be reported, wherein enantioselectivity is more pronounced at D3 than at D2, and that a binding region on the second extracellular loop (E2) may play a role in both enantioselectivity and D3 receptor selectivity. Moreover, we have discovered some of the most D3-selective compounds reported to date that show high affinity (K(i) = 1 nM) for D3 and approximately 400-fold selectivity over the D2 receptor subtype. Several of these analogues showed exquisite selectivity for D3 receptors over >60 other receptors, further underscoring their value as in vivo research tools. These lead compounds also have appropriate physical characteristics for in vivo exploration and therefore will be useful in determining how intrinsic activity at D3 receptors tested in vitro is related to behaviors in animal models of addiction and other neuropsychiatric disorders.
Figures
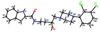
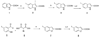

Similar articles
-
Evaluation of N-phenyl homopiperazine analogs as potential dopamine D3 receptor selective ligands.Bioorg Med Chem. 2013 Jun 1;21(11):2988-98. doi: 10.1016/j.bmc.2013.03.074. Epub 2013 Apr 6. Bioorg Med Chem. 2013. PMID: 23618707 Free PMC article.
-
Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents.J Med Chem. 2007 Aug 23;50(17):4135-46. doi: 10.1021/jm0704200. Epub 2007 Aug 2. J Med Chem. 2007. PMID: 17672446
-
Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor.J Med Chem. 2005 Feb 10;48(3):839-48. doi: 10.1021/jm049465g. J Med Chem. 2005. PMID: 15689168
-
The First Negative Allosteric Modulator for Dopamine D2 and D3 Receptors, SB269652 May Lead to a New Generation of Antipsychotic Drugs.Mol Pharmacol. 2017 Jun;91(6):586-594. doi: 10.1124/mol.116.107607. Epub 2017 Mar 6. Mol Pharmacol. 2017. PMID: 28265019 Free PMC article. Review.
-
Bitropic D3 Dopamine Receptor Selective Compounds as Potential Antipsychotics.Curr Pharm Des. 2015;21(26):3700-24. doi: 10.2174/1381612821666150724100830. Curr Pharm Des. 2015. PMID: 26205291 Review.
Cited by
-
Design, synthesis, and structure-activity relationship studies of a series of [4-(4-carboxamidobutyl)]-1-arylpiperazines: insights into structural features contributing to dopamine D3 versus D2 receptor subtype selectivity.J Med Chem. 2014 Aug 28;57(16):7042-60. doi: 10.1021/jm500801r. Epub 2014 Aug 15. J Med Chem. 2014. PMID: 25126833 Free PMC article.
-
Effects of chronic buspirone treatment on nicotine and concurrent nicotine+cocaine self-administration.Neuropsychopharmacology. 2013 Jun;38(7):1264-75. doi: 10.1038/npp.2013.25. Epub 2013 Jan 21. Neuropsychopharmacology. 2013. PMID: 23337868 Free PMC article.
-
The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access.Addict Biol. 2010 Jul;15(3):312-23. doi: 10.1111/j.1369-1600.2010.00211.x. Epub 2010 Apr 29. Addict Biol. 2010. PMID: 20456290 Free PMC article.
-
Ligand discovery from a dopamine D3 receptor homology model and crystal structure.Nat Chem Biol. 2011 Sep 18;7(11):769-78. doi: 10.1038/nchembio.662. Nat Chem Biol. 2011. PMID: 21926995 Free PMC article.
-
New Dopamine D3-Selective Receptor Ligands Containing a 6-Methoxy-1,2,3,4-tetrahydroisoquinolin-7-ol Motif.ACS Med Chem Lett. 2018 Sep 10;9(10):990-995. doi: 10.1021/acsmedchemlett.8b00229. eCollection 2018 Oct 11. ACS Med Chem Lett. 2018. PMID: 30344905 Free PMC article.
References
-
- Joyce JN, Milian MJ. Dopamine D-3 receptor antagonists as therapeutic agents. Drug Discov. Today. 2005;10:917–925. - PubMed
-
- Newman AH, Grundt P, Nader MA. Dopamine D3 Receptor Partial Agonists and Antagonists as Potential Drug Abuse Therapeutic Agents. J. Med. Chem. 2005;11:3663–3679. - PubMed
-
- Newman-Tancredi A, Cussac D, Audinot V, Pasteau V, Gavaudan S, Millan MJ. G protein activation by human dopamine D3 receptors in high expressing Chinese hamster ovary cells: A guanosine-5’-O-(3-[35S]thio)-triphosphate binding and antibody study. Mol. Pharmacol. 1999;55:564–574. - PubMed
-
- Cussac D, Newman-Tancredi A, Pasteau V, Millan MJ. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol. Pharmacol. 1999;56:1025–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

