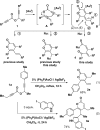
Gold(I)-catalyzed coupling reactions for the synthesis of diverse small molecules using the build/couple/pair strategy
- PMID: 19331418
- PMCID: PMC2669759
- DOI: 10.1021/ja900414s
Gold(I)-catalyzed coupling reactions for the synthesis of diverse small molecules using the build/couple/pair strategy
Abstract
The build/couple/pair strategy has yielded small molecules with stereochemical and skeletal diversity by using short reaction sequences. Subsequent screening has shown that these compounds can achieve biological tasks considered challenging if not impossible ('undruggable') for small molecules. We have developed gold(I)-catalyzed cascade reactions of easily prepared propargyl propiolates as a means to achieve effective intermolecular coupling reactions for this strategy. Sequential alkyne activation of propargyl propiolates by a cationic gold(I) catalyst yields an oxocarbenium ion that we previously showed is trapped by C-based nucleophiles at an extrannular site to yield alpha-pyrones. Here, we report O-based nucleophiles react by ring opening to afford a novel polyfunctional product. In addition, by coupling suitable building blocks, we subsequently performed intramolecular pairing reactions that yield diverse and complex skeletons. These pairing reactions include one based on a novel aza-Wittig-6pi-electrocyclization sequence and others based on ring-closing metathesis reactions.
Figures
Similar articles
-
Syntheses of α-pyrones using gold-catalyzed coupling reactions.Org Lett. 2011 Jun 3;13(11):2834-6. doi: 10.1021/ol200794w. Epub 2011 May 2. Org Lett. 2011. PMID: 21534543 Free PMC article.
-
Gold-catalyzed sequential alkyne activation for the synthesis of 4,6-disubstituted phosphorus 2-pyrones.Org Lett. 2013 Jan 4;15(1):26-9. doi: 10.1021/ol3029274. Epub 2012 Dec 13. Org Lett. 2013. PMID: 23236965
-
Practical, modular, and general synthesis of 3-coumaranones through gold-catalyzed intermolecular alkyne oxidation strategy.Chem Asian J. 2015 Jan;10(1):91-5. doi: 10.1002/asia.201403032. Epub 2014 Oct 6. Chem Asian J. 2015. PMID: 25287758
-
[Development of Novel Preparations for Nitrogen Heterocycles Based on Cascade Reactions].Yakugaku Zasshi. 2018;138(9):1151-1161. doi: 10.1248/yakushi.18-00122. Yakugaku Zasshi. 2018. PMID: 30175759 Review. Japanese.
-
Gold α-oxo carbenoids in catalysis: catalytic oxygen-atom transfer to alkynes.Angew Chem Int Ed Engl. 2011 Aug 1;50(32):7226-36. doi: 10.1002/anie.201100148. Epub 2011 Jul 1. Angew Chem Int Ed Engl. 2011. PMID: 21726021 Review.
Cited by
-
Syntheses of α-pyrones using gold-catalyzed coupling reactions.Org Lett. 2011 Jun 3;13(11):2834-6. doi: 10.1021/ol200794w. Epub 2011 May 2. Org Lett. 2011. PMID: 21534543 Free PMC article.
-
Modular, One-Pot, Sequential Aziridine Ring Opening-S(N)Ar Strategy to 7-, 10-, and 11-Membered Benzo-Fused Sultams.J Org Chem. 2015 Oct 16;80(20):9926-41. doi: 10.1021/acs.joc.5b01429. Epub 2015 Oct 8. J Org Chem. 2015. PMID: 26446396 Free PMC article.
-
An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: discovery of macrocyclic histone deacetylase inhibitors.J Am Chem Soc. 2010 Dec 1;132(47):16962-76. doi: 10.1021/ja105119r. Epub 2010 Nov 10. J Am Chem Soc. 2010. PMID: 21067169 Free PMC article.
-
A modular reaction pairing approach to the diversity-oriented synthesis of fused- and bridged-polycyclic sultams.Org Lett. 2011 Oct 7;13(19):5148-51. doi: 10.1021/ol201962n. Epub 2011 Sep 7. Org Lett. 2011. PMID: 21899284 Free PMC article.
-
A biomimetic polyketide-inspired approach to small-molecule ligand discovery.Nat Chem. 2011 Nov 20;4(2):99-104. doi: 10.1038/nchem.1200. Nat Chem. 2011. PMID: 22270625 Free PMC article.
References
-
- Tolliday N.; Clemons P. A.; Ferraiolo P.; Koehler A. N.; Lewis T. A.; Li X.; Schreiber S. L.; Gerhard D. S.; Eliasof S. Cancer Res. 2006, 66, 8935–8942. - PubMed
- Inglese J.; Johnson R. L.; Simeonov A.; Xia M.; Zheng W.; Austin C. P.; Auld D. S. Nat. Chem. Biol. 2007, 3, 466–479. - PubMed
- Fox S.; Farr-Jones S.; Sopchak L.; Boggs A.; Nicely H. W.; Khoury R.; Biros M. J. Biomol. Screen. 2006, 11, 864–869. - PubMed
-
- Payne D. J.; Gwynn M. N.; Holmes D. J.; Pompliano D. L. Nat. Rev. Drug Discov. 2007, 6, 29–40. - PubMed
-
- Ng P. Y.; Tang Y.; Knosp W. M.; Stadler S.; Shaw J. T. Angew. Chem., Int. Ed. 2007, 46, 5352–5355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources