Brain stress systems in the amygdala and addiction
- PMID: 19332030
- PMCID: PMC2774745
- DOI: 10.1016/j.brainres.2009.03.038
Brain stress systems in the amygdala and addiction
Abstract
Dysregulation of the brain emotional systems that mediate arousal and stress is a key component of the pathophysiology of drug addiction. Drug addiction is a chronically relapsing disorder characterized by a compulsion to seek and take drugs and the development of dependence and manifestation of a negative emotional state when the drug is removed. Activation of brain stress systems is hypothesized to be a key element of the negative emotional state produced by dependence that drives drug-seeking through negative reinforcement mechanisms. The focus of the present review is on the role of two key brain arousal/stress systems in the development of dependence. Emphasis is placed on the neuropharmacological actions of corticotropin-releasing factor (CRF) and norepinephrine in extrahypothalamic systems in the extended amygdala, including the central nucleus of the amygdala, bed nucleus of the stria terminalis, and a transition area in the shell of the nucleus accumbens. Compelling evidence argues that these brain stress systems, a heretofore largely neglected component of dependence and addiction, play a key role in engaging the transition to dependence and maintaining dependence once it is initiated. Understanding the role of the brain stress and anti-stress systems in addiction not only provides insight into the neurobiology of the "dark side" of addiction but also provides insight into the organization and function of basic brain emotional circuitry that guides motivated behavior.
Figures
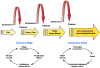

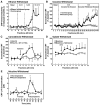

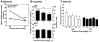

References
-
- Akbarian S, Bates B, Liu RJ, Skirboll SL, Pejchal T, Coppola V, Sun LD, Fan G, Kucera J, Wilson MA, Tessarollo L, Kosofsky BE, Taylor JR, Bothwell M, Nestler EJ, Aghajanian GK, Jaenisch R. Neurotrophin-3 modulates noradrenergic neuron function and opiate withdrawal. Mol Psychiatry. 2001;6:593–604. - PubMed
-
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. - PubMed
-
- Alling C, Balldin J, Bokstrom K, Gottfries CG, Karlsson I, Langstrom G. Studies on duration of a late recovery period after chronic abuse of ethanol: a cross-sectional study of biochemical and psychiatric indicators. Acta Psychiatr Scand. 1982;66:384–397. - PubMed
-
- Alonso G, Szafarczyk A, Balmefrezol M, Assenmacher I. Immunocytochemical evidence for stimulatory control by the ventral noradrenergic bundle of parvocellular neurons of the paraventricular nucleus secreting corticotropin-releasing hormone and vasopressin in rats. Brain Res. 1986;397:297–307. - PubMed

