Differential metabolic effects of glucosamine and N-acetylglucosamine in human articular chondrocytes
- PMID: 19332174
- PMCID: PMC2785807
- DOI: 10.1016/j.joca.2009.03.004
Differential metabolic effects of glucosamine and N-acetylglucosamine in human articular chondrocytes
Abstract
Objective: Aminosugars are commonly used to treat osteoarthritis; however, molecular mechanisms mediating their anti-arthritic activities are still poorly understood. This study analyzes facilitated transport and metabolic effects of glucosamine (GlcN) and N-acetylglucosamine (GlcNAc) in human articular chondrocytes.
Methods: Human articular chondrocytes were isolated from knee cartilage. Facilitated transport of glucose, GlcN and GlcNAc was measured by uptake of [3H]2-deoxyglucose, [3H]GlcN and [3H]GlcNAc. Glucose transporter (GLUT) expression was analyzed by Western blotting. Production of sulfated glycosaminoglycans (SGAG) was measured using [(35)S]SO4. Hyaluronan was quantified using hyaluronan binding protein.
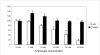
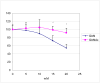
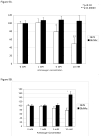
Results: Chondrocytes actively import and metabolize GlcN but not GlcNAc and this represents a cell-type specific phenomenon. Similar to facilitated glucose transport, GlcN transport in chondrocytes is accelerated by cytokines and growth factors. GlcN non-competitively inhibits basal glucose transport, which in part depends on GlcN-mediated depletion of ATP stores. In IL-1beta-stimulated chondrocytes, GlcN inhibits membrane translocation of GLUT1 and 6, but does not affect the expression of GLUT3. In contrast to GlcN, GlcNAc accelerates facilitated glucose transport. In parallel with the opposing actions of these aminosugars on glucose transport, GlcN inhibits hyaluronan and SGAG synthesis while GlcNAc stimulates hyaluronan synthesis. GlcNAc-accelerated hyaluronan synthesis is associated with upregulation of hyaluronan synthase-2.
Conclusion: Differences in GlcN and GlcNAc uptake, and their subsequent effects on glucose transport, GLUT expression and SGAG and hyaluronan synthesis, indicate that these two aminosugars have distinct molecular mechanisms mediating their differential biological activities in chondrocytes.
Figures




Similar articles
-
Glucosamine promotes chondrogenic phenotype in both chondrocytes and mesenchymal stem cells and inhibits MMP-13 expression and matrix degradation.Osteoarthritis Cartilage. 2007 Jun;15(6):646-55. doi: 10.1016/j.joca.2007.01.014. Epub 2007 Mar 6. Osteoarthritis Cartilage. 2007. PMID: 17337215
-
Modulation of articular chondrocyte proliferation and anionic glycoconjugate synthesis by glucosamine (GlcN), N-acetyl GlcN (GlcNAc) GlcN sulfate salt (GlcN.S) and covalent glucosamine sulfates (GlcN-SO4).Osteoarthritis Cartilage. 2007 Aug;15(8):946-56. doi: 10.1016/j.joca.2007.02.010. Epub 2007 Apr 2. Osteoarthritis Cartilage. 2007. PMID: 17400483
-
The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes--implications for osteoarthritis.Biochem Biophys Res Commun. 2011 Feb 18;405(3):362-7. doi: 10.1016/j.bbrc.2011.01.007. Epub 2011 Jan 8. Biochem Biophys Res Commun. 2011. PMID: 21219853 Free PMC article.
-
Biological activities of glucosamine and its related substances.Adv Food Nutr Res. 2012;65:337-52. doi: 10.1016/B978-0-12-416003-3.00022-6. Adv Food Nutr Res. 2012. PMID: 22361198 Review.
-
Recent advancement of glucosamine and N-acetyl glucosamine production using microorganisms: A review.J Ind Microbiol Biotechnol. 2024 Dec 31;52:kuaf014. doi: 10.1093/jimb/kuaf014. J Ind Microbiol Biotechnol. 2024. PMID: 40424515 Free PMC article. Review.
Cited by
-
The Hexosamine Biosynthetic Pathway as a Therapeutic Target after Cartilage Trauma: Modification of Chondrocyte Survival and Metabolism by Glucosamine Derivatives and PUGNAc in an Ex Vivo Model.Int J Mol Sci. 2021 Jul 6;22(14):7247. doi: 10.3390/ijms22147247. Int J Mol Sci. 2021. PMID: 34298867 Free PMC article.
-
Lipopolysaccharide (LPS)-stimulated iNOS Induction Is Increased by Glucosamine under Normal Glucose Conditions but Is Inhibited by Glucosamine under High Glucose Conditions in Macrophage Cells.J Biol Chem. 2017 Feb 3;292(5):1724-1736. doi: 10.1074/jbc.M116.737940. Epub 2016 Dec 7. J Biol Chem. 2017. PMID: 27927986 Free PMC article.
-
Evaluation of the chondroprotective action of N-acetylglucosamine in a rat experimental osteoarthritis model.Exp Ther Med. 2017 Oct;14(4):3137-3144. doi: 10.3892/etm.2017.4849. Epub 2017 Jul 28. Exp Ther Med. 2017. PMID: 28912864 Free PMC article.
-
Evaluation of the effect of N-acetyl-glucosamine administration on biomarkers for cartilage metabolism in healthy individuals without symptoms of arthritis: A randomized double-blind placebo-controlled clinical study.Exp Ther Med. 2016 Sep;12(3):1481-1489. doi: 10.3892/etm.2016.3480. Epub 2016 Jun 24. Exp Ther Med. 2016. PMID: 27588069 Free PMC article.
-
Effect of N-acetylglucosamine administration on cartilage metabolism and safety in healthy subjects without symptoms of arthritis: A case report.Exp Ther Med. 2017 Apr;13(4):1614-1621. doi: 10.3892/etm.2017.4140. Epub 2017 Feb 21. Exp Ther Med. 2017. PMID: 28413518 Free PMC article.
References
-
- Dieppe P, Chard J, Lohmnader S, Smith C. Osteoarthritis. Clin Evid. 2002;7:1091–90. - PubMed
-
- Reginster JY. The prevalence and burden of arthritis. Rheumatology (Oxford) 2002;41:3–6. - PubMed
-
- Goldberg SH, Von Feldt JM, Lonner JH. Pharmacologic therapy for osteoarthritis. Am J Orthop. 2002;31:673–80. - PubMed
-
- Rubin BR, Talent JM, Kongtawelert P, Pertusi RM, Forman MD, Gracy RW. Oral polymeric N-acetyl-D-glucosamine and osteoarthritis. J Am Osteopath Assoc. 2001;101:339–44. - PubMed
-
- Christgau S, Henrotin Y, Tanko LB, Rovati LC, Collette J, Bruyere O, et al. Osteoarthritic patients with high cartilage turnover show increased responsiveness to the cartilage protecting effects of glucosamine sulphate. Clin Exp Rheumatol. 2004;22:36–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

