Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction
- PMID: 19332462
- PMCID: PMC2740924
- DOI: 10.1161/CIRCULATIONAHA.108.823799
Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction
Retraction in
-
Retraction of: Transgenic Overexpression of Aldehyde Dehydrogenase-2 Rescues Chronic Alcohol Intake-Induced Myocardial Hypertrophy and Contractile Dysfunction.Circulation. 2023 Sep 26;148(13):e147. doi: 10.1161/CIR.0000000000001083. Epub 2022 Jul 22. Circulation. 2023. PMID: 35866413 Free PMC article. No abstract available.
Abstract
Background: Chronic alcoholism leads to the onset and progression of alcoholic cardiomyopathy through toxic mechanisms of ethanol and its metabolite, acetaldehyde. This study examined the impact of altered acetaldehyde metabolism through systemic transgenic overexpression of aldehyde dehydrogenase-2 (ALDH2) on chronic alcohol ingestion-induced myocardial damage.
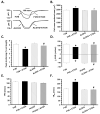
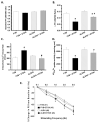
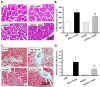
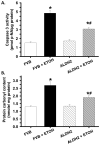
Methods and results: ALDH2 transgenic mice were produced with the chicken beta-actin promoter. Wild-type FVB and ALDH2 mice were placed on a 4% alcohol diet or a control diet for 14 weeks. Myocardial and cardiomyocyte contraction, intracellular Ca(2+) handling, histology (hematoxylin and eosin, Masson trichrome), protein damage, and apoptosis were determined. Western blot was used to monitor the expression of NADPH oxidase, calcineurin, apoptosis-stimulated kinase (ASK-1), glycogen synthase kinase-3beta (GSK-3beta), GATA4, and cAMP-response element binding (CREB) protein. ALDH2 reduced the chronic alcohol ingestion-induced elevation in plasma and tissue acetaldehyde levels. Chronic alcohol consumption led to cardiac hypertrophy, reduced fractional shortening, cell shortening, and impaired intracellular Ca(2+) homeostasis, the effect of which was alleviated by ALDH2. In addition, the ALDH2 transgene significantly attenuated chronic alcohol intake-induced myocardial fibrosis, protein carbonyl formation, apoptosis, enhanced NADPH oxidase p47(phox) and calcineurin expression, as well as phosphorylation of ASK-1, GSK-3beta, GATA4, and CREB.
Conclusions: The present results suggest that transgenic overexpression of ALDH2 effectively antagonizes chronic alcohol intake-elicited myocardial hypertrophy and contractile defect through a mechanism that is associated, at least in part, with phosphorylation of ASK-1, GSK-3beta, GATA4, and CREB. These data strongly support the notion that acetaldehyde may be an essential contributor to the chronic development of alcoholic cardiomyopathy.
Figures


References
-
- Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Rubin E, Urbano-Marquez A. The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med. 1994 April 1;120:529–36. - PubMed
-
- Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004 April;32:175–86. - PubMed
-
- Preedy VR, Patel VB, Reilly ME, Richardson PJ, Falkous G, Mantle D. Oxidants, antioxidants and alcohol: implications for skeletal and cardiac muscle. Front Biosci. 1999 August 1;4:e58–e66. - PubMed
-
- Siddiq T, Richardson PJ, Mitchell WD, Teare J, Preedy VR. Ethanol-induced inhibition of ventricular protein synthesis in vivo and the possible role of acetaldehyde. Cell Biochem Funct. 1993 March;11:45–54. - PubMed
-
- Ren J. Acetaldehyde and alcoholic cardiomyopathy: lessons from the ADH and ALDH2 transgenic models. Novartis Found Symp. 2007;285:69–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

