Phospholipase D1 regulates lymphocyte adhesion via upregulation of Rap1 at the plasma membrane
- PMID: 19332557
- PMCID: PMC2698734
- DOI: 10.1128/MCB.00366-09
Phospholipase D1 regulates lymphocyte adhesion via upregulation of Rap1 at the plasma membrane
Abstract
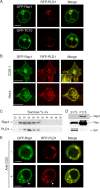
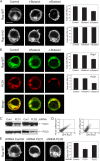
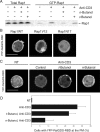
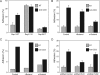
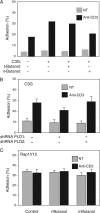
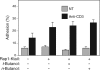
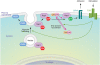
Rap1 is a small GTPase that modulates adhesion of T cells by regulating inside-out signaling through LFA-1. The bulk of Rap1 is expressed in a GDP-bound state on intracellular vesicles. Exocytosis of these vesicles delivers Rap1 to the plasma membrane, where it becomes activated. We report here that phospholipase D1 (PLD1) is expressed on the same vesicular compartment in T cells as Rap1 and is translocated to the plasma membrane along with Rap1. Moreover, PLD activity is required for both translocation and activation of Rap1. Increased T-cell adhesion in response to stimulation of the antigen receptor depended on PLD1. C3G, a Rap1 guanine nucleotide exchange factor located in the cytosol of resting cells, translocated to the plasma membranes of stimulated T cells. Our data support a model whereby PLD1 regulates Rap1 activity by controlling exocytosis of a stored, vesicular pool of Rap1 that can be activated by C3G upon delivery to the plasma membrane.
Figures

Similar articles
-
Cytotoxic-T-lymphocyte antigen 4 receptor signaling for lymphocyte adhesion is mediated by C3G and Rap1.Mol Cell Biol. 2014 Mar;34(6):978-88. doi: 10.1128/MCB.01024-13. Epub 2014 Jan 6. Mol Cell Biol. 2014. PMID: 24396067 Free PMC article.
-
The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1.J Cell Biol. 2008 Sep 22;182(6):1231-44. doi: 10.1083/jcb.200801121. J Cell Biol. 2008. PMID: 18809728 Free PMC article.
-
Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis.EMBO J. 2001 Jul 2;20(13):3333-41. doi: 10.1093/emboj/20.13.3333. EMBO J. 2001. PMID: 11432821 Free PMC article.
-
C3G Protein, a New Player in Glioblastoma.Int J Mol Sci. 2021 Sep 16;22(18):10018. doi: 10.3390/ijms221810018. Int J Mol Sci. 2021. PMID: 34576182 Free PMC article. Review.
-
Regulation of lymphocyte adhesion and migration by the small GTPase Rap1 and its effector molecule, RAPL.Immunol Lett. 2004 Apr 30;93(1):1-5. doi: 10.1016/j.imlet.2004.02.008. Immunol Lett. 2004. PMID: 15134891 Review.
Cited by
-
Cytosolic Ras supports eye development in Drosophila.Mol Cell Biol. 2010 Dec;30(24):5649-57. doi: 10.1128/MCB.00635-10. Epub 2010 Oct 11. Mol Cell Biol. 2010. PMID: 20937772 Free PMC article.
-
R-Ras Regulates Murine T Cell Migration and Intercellular Adhesion Molecule-1 Binding.PLoS One. 2015 Dec 28;10(12):e0145218. doi: 10.1371/journal.pone.0145218. eCollection 2015. PLoS One. 2015. PMID: 26710069 Free PMC article.
-
The Rap1-RIAM-talin axis of integrin activation and blood cell function.Blood. 2016 Jul 28;128(4):479-87. doi: 10.1182/blood-2015-12-638700. Epub 2016 May 20. Blood. 2016. PMID: 27207789 Free PMC article. Review.
-
T Lymphocyte Migration: An Action Movie Starring the Actin and Associated Actors.Front Immunol. 2015 Nov 18;6:586. doi: 10.3389/fimmu.2015.00586. eCollection 2015. Front Immunol. 2015. PMID: 26635800 Free PMC article. Review.
-
PLCε1 regulates SDF-1α-induced lymphocyte adhesion and migration to sites of inflammation.Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2693-2698. doi: 10.1073/pnas.1612900114. Epub 2017 Feb 17. Proc Natl Acad Sci U S A. 2017. PMID: 28213494 Free PMC article.
References
-
- Amsen, D., A. Kruisbeek, J. L. Bos, and K. Reedquist. 2000. Activation of the Ras-related GTPase Rap1 by thymocyte TCR engagement and during selection. Eur. J. Immunol. 302832-2841. - PubMed
-
- Bi, K., M. G. Roth, and N. T. Ktistakis. 1997. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr. Biol. 7301-307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
