Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab
- PMID: 19332770
- PMCID: PMC2765344
- DOI: 10.1215/15228517-2009-006
Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab
Abstract
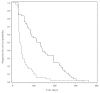
Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that has efficacy in recurrent malignant gliomas, particularly in combination with irinotecan. However, responses are rarely durable. Continuation of bevacizumab in combination with another chemotherapeutic agent may demonstrate some activity. In this article we present a retrospective review of 54 patients with recurrent malignant gliomas who progressed on a bevacizumab-containing regimen and were then treated with an alternate bevacizumab-containing regimen. All patients received intravenous bevacizumab (5-10 mg/kg) every 2 weeks alone or in combination with an additional chemotherapeutic agent, such as irinotecan. There was no limit on the number of prior therapies. Clinical characteristics and outcomes were reviewed. Tumor progression was determined by a combination of clinical status and radiographic changes. Patients were 33 men, 21 women (median age, 50 years; range, 23-72 years) with a median KPS score of 80 prior to the first bevacizumab-containing regimen and 70 prior to the second regimen; median prior chemotherapy regimens including the first bevacizumab-containing regimen was 3 (range, 2-5). Median progression-free survival (PFS) on the first bevacizumab-containing regimen was 124 days (95% confidence interval [CI], 87-154 days); 6-month (6M)-PFS was 33%. Median PFS on the second bevacizumab-containing regimen was 37.5 days (95% CI, 34-42 days); 6M-PFS was 2%. Ten patients on the first regimen and 12 patients on the second regimen suffered grade 3/4 toxicities. Those patients with malignant gliomas who progressed despite a bevacizumab-containing regimen rarely responded to the second bevacizumab-containing chemotherapeutic regimen. In such patients, alternate therapies should be considered.
Figures
References
-
- Brandsma D, van den Bent MJ. Molecular targeted therapies and chemotherapy in malignant gliomas. Curr Opin Oncol. 2007;19:598–605. - PubMed
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. - PubMed
-
- Cloughesy T, Prados M, Wen PY, et al. A phase II, randomized, non-comparative clinical trial of bevacizumab alone or in combination with irinotecan prolongs 6-month PFS in recurrent, treatment-refractory glioblastoma [abstract] J Clin Oncol. 2008;26:2010b.
-
- Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. - PubMed
-
- Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical