Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells
- PMID: 19332787
- PMCID: PMC2669345
- DOI: 10.1073/pnas.0810799106
Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells
Abstract
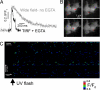
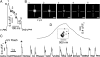
The spatiotemporal patterning of Ca(2+) signals regulates numerous cellular functions, and is determined by the functional properties and spatial clustering of inositol trisphosphate receptor (IP(3)R) Ca(2+) release channels in the endoplasmic reticulum membrane. However, studies at the single-channel level have been hampered because IP(3)Rs are inaccessible to patch-clamp recording in intact cells, and because excised organelle and bilayer reconstitution systems disrupt the Ca(2+)-induced Ca(2+) release (CICR) process that mediates channel-channel coordination. We introduce here the use of total internal reflection fluorescence microscopy to image single-channel Ca(2+) flux through individual and clustered IP(3)Rs in intact mammalian cells. This enables a quantal dissection of the local calcium puffs that constitute building blocks of cellular Ca(2+) signals, revealing stochastic recruitment of, on average, approximately 6 active IP(3)Rs clustered within <500 nm. Channel openings are rapidly ( approximately 10 ms) recruited by opening of an initial trigger channel, and a similarly rapid inhibitory process terminates puffs despite local [Ca(2+)] elevation that would otherwise sustain Ca(2+)-induced Ca(2+) release indefinitely. Minimally invasive, nano-scale Ca(2+) imaging provides a powerful tool for the functional study of intracellular Ca(2+) release channels while maintaining the native architecture and dynamic interactions essential for discrete and selective cell signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. - PubMed
-
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic calcium oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993;74:661–668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

