Angiogenin cleaves tRNA and promotes stress-induced translational repression
- PMID: 19332886
- PMCID: PMC2700517
- DOI: 10.1083/jcb.200811106
Angiogenin cleaves tRNA and promotes stress-induced translational repression
Abstract
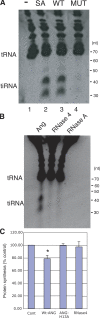
Stress-induced phosphorylation of eIF2alpha inhibits global protein synthesis to conserve energy for repair of stress-induced damage. Stress-induced translational arrest is observed in cells expressing a nonphosphorylatable eIF2alpha mutant (S51A), which indicates the existence of an alternative pathway of translational control. In this paper, we show that arsenite, heat shock, or ultraviolet irradiation promotes transfer RNA (tRNA) cleavage and accumulation of tRNA-derived, stress-induced small RNAs (tiRNAs). We show that angiogenin, a secreted ribonuclease, is required for stress-induced production of tiRNAs. Knockdown of angiogenin, but not related ribonucleases, inhibits arsenite-induced tiRNA production and translational arrest. In contrast, knockdown of the angiogenin inhibitor RNH1 enhances tiRNA production and promotes arsenite-induced translational arrest. Moreover, recombinant angiogenin, but not RNase 4 or RNase A, induces tiRNA production and inhibits protein synthesis in the absence of exogenous stress. Finally, transfection of angiogenin-induced tiRNAs promotes phospho-eIF2alpha-independent translational arrest. Our results introduce angiogenin and tiRNAs as components of a phospho-eIF2alpha-independent stress response program.
Figures



References
-
- Anderson P., Kedersha N. 2008. Stress granules: the Tao of RNA triage.Trends Biochem. Sci. 33:141–150 - PubMed
-
- Ardelt W., Mikulski S.M., Shogen K. 1991. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. Homology to pancreatic ribonucleases.J. Biol. Chem. 266:245–251 - PubMed
-
- Brennecke J., Aravin A.A., Stark A., Dus M., Kellis M., Sachidanandam R., Hannon G.J. 2007. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila.Cell. 128:1089–1103 - PubMed
-
- Fett J.W., Strydom D.J., Lobb R.R., Alderman E.M., Bethune J.L., Riordan J.F., Vallee B.L. 1985. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells.Biochemistry. 24:5480–5486 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

