Patient-assessed versus physician-assessed disease severity and outcome in patients with nonspecific pain associated with major depressive disorder
- PMID: 19333404
- PMCID: PMC2660158
- DOI: 10.4088/pcc.08m00670
Patient-assessed versus physician-assessed disease severity and outcome in patients with nonspecific pain associated with major depressive disorder
Abstract
Objectives: This post hoc analysis compared how patients and physicians estimate disease severity and global improvement during 8 weeks of treatment for major depressive disorder (MDD) with associated nonspecific pain. In addition, predictors of pain and depression were identified.
Method: Data were derived from a double-blind, placebo-controlled, multicenter, European study (conducted from May 2005 to May 2006) in adult outpatients with MDD (DSM-IV criteria) and moderate pain not attributable to a diagnosed organic pain syndrome (Brief Pain Inventory-Short Form [BPI-SF] average pain score ≥ 3). Patients were randomly assigned to duloxetine 60 mg/day or placebo and treated for 8 weeks. Physicians were asked to rate severity of depression by using the Montgomery-Asberg Depression Rating Scale (MADRS) and the Clinical Global Impressions-Severity of Illness (CGI-S) and CGI-Improvement (CGI-I) scales. Patients were asked to assess pain using the BPI-SF, psychological symptomatology (9 domains including depression) with the Symptom Checklist-90-Revised (SCL-90-R), and overall improvement with the Patient Global Impression of Improvement (PGI-I). Multivariate linear regressions were performed as post hoc analyses to identify predictors of disease assessment at baseline and at the end of the study using a last-observation-carried-forward approach.
Results: All SCL-90-R domains improved during the 8 weeks of treatment. At baseline, the MADRS was associated only with the SCL-90-R obsessive-compulsive score, while the SCL-90-R depression score was associated with the BPI-SF average pain score and with many SCL-90-R subscores. The global impression of improvement was rated higher by the physicians than by the patients. At the end of the study, CGI-I was significantly associated with a decrease in depression severity (MADRS; p < .0001), younger age (p = .0005), and a decrease of the SCL-90-R interpersonal sensitivity score (p = .0359), but not with BPI-SF average pain. In contrast, patient-rated PGI-I was significantly associated with the SCL-90-R depressive domain (p < .0001), BPI-SF average pain (p = .0003), and the SCL-90-R anxiety domain (p = .0041) scores.
Conclusion: In patients with MDD associated with at least moderate nonspecific pain, physicians consider mainly the change in depressive symptoms as measured by MADRS in their CGI-I ratings, while patients also consider pain, depression, and anxiety in their PGI-I ratings. When treating depression and assessing treatment outcome, a broad spectrum of symptoms needs to be monitored.
Trial registration: clinicaltrials.gov Identifier: NCT00191919.
Figures
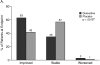
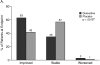
References
-
- Guy W. Rockville, Md: National Institute of Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338; pp. 218–222.
-
- Lehman AF, Babigian HM, Reed SK. The epidemiology of treatment for chronic and nonchronic mental disorders. J Nerv Ment Dis. 1984;172:658–666. - PubMed
-
- Beneke M, Rasmus W. “Clinical Global Impressions” (ECDEU): some critical comments. Pharmacopsychiatry. 1991;25(4):171–176. - PubMed
-
- Leon AC, Shear MK, Klerman GL, et al. A comparison of symptom determinants of patient and clinician global ratings in patients with panic disorder and depression. J Clin Psychopharmacol. 1993;13:327–331. - PubMed
-
- Zaider TI, Heimberg RG, Fresco DM, et al. Evaluation of the Clinical Global Impressions scale among individuals with social anxiety disorder. Psychol Med. 2003;33:611–622. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
