Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis
- PMID: 19334059
- PMCID: PMC2666109
- DOI: 10.1002/ana.21611
Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis
Abstract
Objective: Virus infections are the most common causes of encephalitis, a syndrome characterized by acute inflammation of the brain. More than 150 different viruses have been implicated in the pathogenesis of encephalitis; however, because of limitations with diagnostic testing, causative factors of more than half of the cases remain unknown.
Methods: To investigate whether human herpesvirus-6 (HHV-6) is a causative agent of encephalitis, we examined for evidence of virus infection by determining the presence of viral sequence using polymerase chain reaction and assessed HHV-6 antibody reactivity in the cerebrospinal fluid of encephalitis patients with unknown cause. In a cohort study, we compared virus-specific antibody levels in cerebrospinal fluid samples of patients with encephalitis, relapsing-remitting multiple sclerosis, and other neurological diseases.
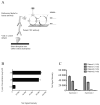
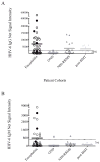
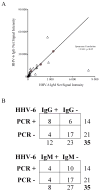
Results: Our results demonstrated increased levels of HHV-6 IgG, as well as IgM levels, in a subset of encephalitis patients compared with other neurological diseases. Moreover, cell-free viral DNA that is indicative of active infection was detected in 40% (14/35) of encephalitis patients, whereas no amplifiable viral sequence was found in either relapsing-remitting MS or other neurological diseases patients. In addition, a significant correlation between polymerase chain reaction detection and anti-HHV-6 antibody response was also demonstrated.
Interpretation: Collectively, these results suggested HHV-6 as a possible pathogen in a subset of encephalitis cases.
Figures


Comment in
-
Diagnosing encephalitis? Consider human herpesvirus type 6.Ann Neurol. 2009 Mar;65(3):235-7. doi: 10.1002/ana.21635. Ann Neurol. 2009. PMID: 19334056 No abstract available.
References
-
- Glaser CA, Honarmand S, Anderson LJ, et al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43:1565–1577. - PubMed
-
- Glaser CA, Gilliam S, Schnurr D, et al. In search of encephalitis etiologies: diagnostic challenges in the California Encephalitis Project, 1998–2000. Clin Infect Dis. 2003;36:731–742. - PubMed
-
- Lusso P. HHV-6 and the immune system: mechanisms of immunomodulation and viral escape. J Clin Virol. 2006;37(Suppl 1):S4–10. - PubMed
-
- Froberg MK. Review: CMV escapes! Ann Clin Lab Sci. 2004;34:123–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

