Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases
- PMID: 19335094
- PMCID: PMC2848511
- DOI: 10.1089/ars.2008.2368
Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases
Abstract
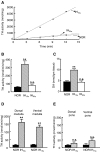
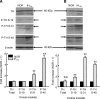
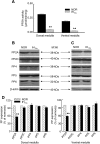
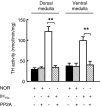
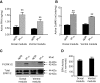
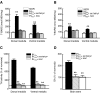
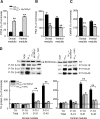
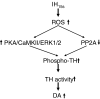
We investigated the role of protein phosphatases (PP) and protein kinases in tyrosine hydroxylase (TH) activation by two patterns of intermittent hypoxia (IH) in rat brainstem. Rats exposed to either IH(15s) (15 s, 5% O(2); 5 min, 21%O(2)) or IH(90s) (90 s each of 10% O(2) & 21%O(2)) for 10 days were used. IH(15s) but not IH(90s) caused a robust increase in TH activity, dopamine (DA) level, and TH phosphorylation at Ser-31 and Ser-40 in the medulla but not in the pons. Likewise, IH(15s) but not IH(90s) decreased activity and expression of protein phosphatase 2A (PP2A) and increased activity of multiple protein kinases. In vitro dephosphorylation with PP2A nearly abolished IH(15s)-induced increase in TH activity. IH(15s) increased generation of reactive oxygen species (ROS) in brainstem medullary regions which was nearly threefold higher than that evoked by IH(90s). Antioxidants prevented IH(15s)-induced downregulation of PP2A and increases in multiple protein kinase activity with subsequent reversal of serine phosphorylation of TH, TH activity, and DA to control levels. These findings demonstrate that IH in a pattern-specific manner activates TH involving ROS-mediated sustained increase in TH phosphorylation via downregulation of PP2A and upregulation of protein kinases.
Figures
Similar articles
-
Activation of tyrosine hydroxylase by intermittent hypoxia: involvement of serine phosphorylation.J Appl Physiol (1985). 2003 Aug;95(2):536-44. doi: 10.1152/japplphysiol.00186.2003. Epub 2003 Apr 11. J Appl Physiol (1985). 2003. PMID: 12692140
-
Intermittent hypoxia activates peptidylglycine alpha-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing.J Appl Physiol (1985). 2009 Jan;106(1):12-9. doi: 10.1152/japplphysiol.90702.2008. Epub 2008 Sep 25. J Appl Physiol (1985). 2009. PMID: 18818385 Free PMC article.
-
Inhibition of protein kinase (PK) Cδ attenuates methamphetamine-induced dopaminergic toxicity via upregulation of phosphorylation of tyrosine hydroxylase at Ser40 by modulation of protein phosphatase 2A and PKA.Clin Exp Pharmacol Physiol. 2015 Feb;42(2):192-201. doi: 10.1111/1440-1681.12341. Clin Exp Pharmacol Physiol. 2015. PMID: 25400014
-
Differential regulation of tyrosine hydroxylase by continuous and intermittent hypoxia.Adv Exp Med Biol. 2012;758:381-5. doi: 10.1007/978-94-007-4584-1_51. Adv Exp Med Biol. 2012. PMID: 23080186 Review.
-
Cellular mechanisms associated with intermittent hypoxia.Essays Biochem. 2007;43:91-104. doi: 10.1042/BSE0430091. Essays Biochem. 2007. PMID: 17705795 Review.
Cited by
-
Lasting mesothalamic dopamine imbalance and altered exploratory behavior in rats after a mild neonatal hypoxic event.Front Integr Neurosci. 2024 Jan 17;17:1304338. doi: 10.3389/fnint.2023.1304338. eCollection 2023. Front Integr Neurosci. 2024. PMID: 38304737 Free PMC article.
-
Enhanced neuropeptide Y synthesis during intermittent hypoxia in the rat adrenal medulla: role of reactive oxygen species-dependent alterations in precursor peptide processing.Antioxid Redox Signal. 2011 Apr 1;14(7):1179-90. doi: 10.1089/ars.2010.3353. Epub 2011 Feb 6. Antioxid Redox Signal. 2011. PMID: 20836657 Free PMC article.
-
Intermittent hypoxia-induced protein phosphatase 2A activation reduces PC12 cell proliferation and differentiation.J Biomed Sci. 2014 May 16;21(1):46. doi: 10.1186/1423-0127-21-46. J Biomed Sci. 2014. PMID: 24885237 Free PMC article.
-
Chronic intermittent hypoxia increases rat sternohyoid muscle NADPH oxidase expression with attendant modest oxidative stress.Front Physiol. 2015 Jan 30;6:15. doi: 10.3389/fphys.2015.00015. eCollection 2015. Front Physiol. 2015. PMID: 25688214 Free PMC article.
-
Sympatho-adrenal activation by chronic intermittent hypoxia.J Appl Physiol (1985). 2012 Oct 15;113(8):1304-10. doi: 10.1152/japplphysiol.00444.2012. Epub 2012 Jun 21. J Appl Physiol (1985). 2012. PMID: 22723632 Free PMC article. Review.
References
-
- Ames MM. Lerner P. Lovenberg W. Tyrosine hydroxylase. Activation by protein phosphorylation and end product inhibition. J Biol Chem. 1978;253:27–31. - PubMed
-
- Berresheim U. Kuhn DM. Dephosphorylation of tyrosine hydroxylase by brain protein phosphatases: a predominant role for type 2A. Brain Res. 1994;637:273–276. - PubMed
-
- Bevilaqua LR. Cammarota M. Dickson PW. Sim AT. Dunkley PR. Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J Neurochem. 2003;85:1368–1373. - PubMed
-
- Bobrovskaya L. Gilligan C. Bolster EK. Flaherty JJ. Dickson PW. Dunkley PR. Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem. 2007;100:479–489. - PubMed
-
- Campbell DG. Hardie DG. Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261:10489–10492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

