Substance P release and neurokinin 1 receptor activation in the rat spinal cord increase with the firing frequency of C-fibers
- PMID: 19336248
- PMCID: PMC2692762
- DOI: 10.1016/j.neuroscience.2009.03.058
Substance P release and neurokinin 1 receptor activation in the rat spinal cord increase with the firing frequency of C-fibers
Abstract
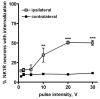
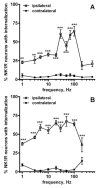
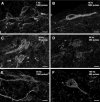
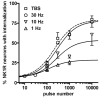
Both the firing frequency of primary afferents and neurokinin 1 receptor (NK1R) internalization in dorsal horn neurons increase with the intensity of noxious stimulus. Accordingly, we studied how the pattern of firing of primary afferent influences NK1R internalization. In rat spinal cord slices, electrical stimulation of the dorsal root evoked NK1R internalization in lamina I neurons by inducing substance P release from primary afferents. The stimulation frequency had pronounced effects on NK1R internalization, which increased up to 100 Hz and then diminished abruptly at 200 Hz. Peptidase inhibitors increased NK1R internalization at frequencies below 30 Hz, indicating that peptidases limit the access of substance P to the receptor at moderate firing rates. NK1R internalization increased with number of pulses at all frequencies, but maximal internalization was substantially lower at 1-10 Hz than at 30 Hz. Pulses organized into bursts produced the same NK1R internalization as sustained 30 Hz stimulation. To determine whether substance P release induced at high stimulation frequencies was from C-fibers, we recorded compound action potentials in the sciatic nerve of anesthetized rats. We observed substantial NK1R internalization when stimulating at intensities evoking a C-elevation, but not at intensities evoking only an Adelta-elevation. Each pulse in trains at frequencies up to 100 Hz evoked a C-elevation, demonstrating that C-fibers can follow these high frequencies. C-elevation amplitudes declined progressively with increasing stimulation frequency, which was likely caused by a combination of factors including temporal dispersion. In conclusion, the instantaneous firing frequency in C-fibers determines the amount of substance P released by noxious stimuli.
Figures





References
-
- Adelson DW, Wei JY, Kruger L. H2O2 sensitivity of afferent splanchnic C fiber units in vitro. J Neurophysiol. 1996;76:371–380. - PubMed
-
- Adelson DW, Wei JY, Kruger L. Warm-sensitive afferent splanchnic C-fiber units in vitro. J Neurophysiol. 1997;77:2989–3002. - PubMed
-
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

