Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells
- PMID: 19336571
- PMCID: PMC2669844
- DOI: 10.1158/0008-5472.CAN-08-4344
Atg5 regulates phenethyl isothiocyanate-induced autophagic and apoptotic cell death in human prostate cancer cells
Abstract
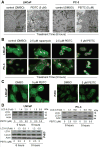
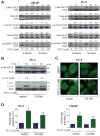
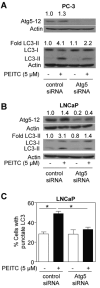
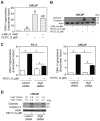
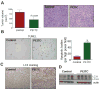
Phenethyl isothiocyanate (PEITC) is a promising cancer chemopreventive agent but the mechanism of its anticancer effect is not fully understood. We now show, for the first time, that PEITC treatment triggers Atg5-dependent autophagic and apoptotic cell death in human prostate cancer cells. Exposure of PC-3 (androgen independent, p53 null) and LNCaP (androgen responsive, wild-type p53) human prostate cancer cells to PEITC resulted in several specific features characteristic of autophagy, including appearance of membranous vacuoles, formation of acidic vesicular organelles, and cleavage and recruitment of microtubule-associated protein 1 light chain 3 (LC3) to autophagosomes. A normal human prostate epithelial cell line (PrEC) was markedly more resistant toward PEITC-mediated cleavage and recruitment of LC3 compared with prostate cancer cells. Although PEITC treatment suppressed activating phosphorylations of Akt and mammalian target of rapamycin (mTOR), which are implicated in regulation of autophagy by different stimuli, processing and recruitment of LC3 was only partially/marginally reversed by ectopic expression of constitutively active Akt or overexpression of mTOR-positive regulator Rheb. The PEITC-mediated apoptotic DNA fragmentation was significantly attenuated in the presence of a pharmacologic inhibitor of autophagy (3-methyl adenine). Transient transfection of LNCaP and PC-3 cells with Atg5-specific small interfering RNA conferred significant protection against PEITC-mediated autophagy as well as apoptotic DNA fragmentation. A xenograft model using PC-3 cells and Caenorhabditis elegans expressing a lgg-1:GFP fusion protein provided evidence for occurrence of PEITC-induced autophagy in vivo. In conclusion, the present study indicates that Atg5 plays an important role in regulation of PEITC-induced autophagic and apoptotic cell death.
Figures

References
-
- Nelson WG, De Marzo AM, Isaacs WB. Prostate Cancer. N Engl J Med. 2003;349:366–81. - PubMed
-
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. - PubMed
-
- Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. - PubMed
-
- Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. - PubMed
-
- Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

