Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study
- PMID: 19336679
- PMCID: PMC2682689
- DOI: 10.2337/db08-0896
Reduced-function SLC22A1 polymorphisms encoding organic cation transporter 1 and glycemic response to metformin: a GoDARTS study
Abstract
Objective: Metformin is actively transported into the liver by the organic cation transporter (OCT)1 (encoded by SLC22A1). In 12 normoglycemic individuals, reduced-function variants in SLC22A1 were shown to decrease the ability of metformin to reduce glucose excursion in response to oral glucose. We assessed the effect of two common loss-of-function polymorphisms in SLC22A1 on metformin response in a large cohort of patients with type 2 diabetes.
Research design and methods: The Diabetes Audit and Research in Tayside Scotland (DARTS) database includes prescribing and biochemistry information and clinical phenotypes of all patients with diabetes within Tayside, Scotland, from 1992 onwards. R61C and 420del variants of SLC22A1 were genotyped in 3,450 patients with type 2 diabetes who were incident users of metformin. We assessed metformin response by modeling the maximum A1C reduction in 18 months after starting metformin and investigated whether a treatment target of A1C <7% was achieved. Sustained metformin effect on A1C between 6 and 42 months was also assessed, as was the time to metformin monotherapy failure. Covariates were SLC22A1 genotype, BMI, average drug dose, adherence, and creatinine clearance.
Results: A total of 1,531 patients were identified with a definable metformin response. R61C and 420del variants did not affect the initial A1C reduction (P = 0.47 and P = 0.92, respectively), the chance of achieving a treatment target (P = 0.83 and P = 0.36), the average A1C on monotherapy up to 42 months (P = 0.44 and P = 0.75), or the hazard of monotherapy failure (P = 0.85 and P = 0.56).
Conclusions: The SLC22A1 loss-of-function variants, R61C and 420del, do not attenuate the A1C reduction achieved by metformin in patients with type 2 diabetes.
Figures

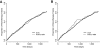
References
-
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B: Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29: 1963– 1972 - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352: 854– 865 - PubMed
-
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL: Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997; 103: 491– 497 - PubMed
-
- Donnelly LA, Doney AS, Hattersley AT, Morris AD, Pearson ER: The effect of obesity on glycaemic response to metformin or sulphonylureas in Type 2 diabetes. Diabet Med 2006; 23: 128– 133 - PubMed
-
- DeFronzo RA, Goodman AM: Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group N Engl J Med 1995; 333: 541– 549 - PubMed

