Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia
- PMID: 19336760
- PMCID: PMC2686193
- DOI: 10.1182/blood-2008-09-179572
Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia
Abstract
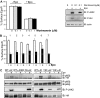
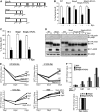
Epo-induced endocytosis of EpoR plays important roles in the down-regulation of EpoR signaling and is the primary means that regulates circulating Epo concentrations. Here we show that cell-surface EpoR is internalized via clathrin-mediated endocytosis. Both JAK2 kinase activity and EpoR cytoplasmic tyrosines are important for ligand-dependent EpoR internalization. Phosphorylated Y429, Y431, and Y479 in the EpoR cytoplasmic domain bind p85 subunit of PI3 kinase on Epo stimulation and individually are sufficient to mediate Epo-dependent EpoR internalization. Knockdown of p85alpha and p85beta or expression of their dominant-negative forms, but not inhibition of PI3 kinase activity, dramatically impaired EpoR internalization, indicating that p85alpha and p85beta may recruit proteins in the endocytic machinery on Epo stimulation. Furthermore, mutated EpoRs from primary familial and congenital polycythemia (PFCP) patients lacking the 3 important tyrosines do not bind p85 or internalize on stimulation. Addition of residues encompassing Y429 and Y431 to these truncated receptors restored p85beta binding and Epo sensitivity. Our results identify a novel PI3 kinase activity-independent function of p85 in EpoR internalization and support a model that defects of internalization in truncated EpoRs from PFCP patients contribute to Epo hypersensitivity and prolonged signaling.
Figures

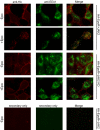
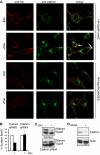
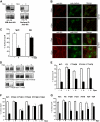
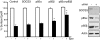
References
-
- Constantinescu SN, Ghaffari S, Lodish HF. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends Endocrinol Metab. 1999;10:18–23. - PubMed
-
- Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. - PubMed
-
- Bouscary D, Pene F, Claessens YE, et al. Critical role for PI 3-kinase in the control of erythropoietin-induced erythroid progenitor proliferation. Blood. 2003;101:3436–3443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

